
While we saw several rising cases of AKI associated with COVID-19, the ESKD population was also vulnerable to this virus. With COVID-19, we didn't know if we would see worsening effects on ESRD or beneficial ( given a not so robust immune system in ESRD). But the proximity and being in a closed dialysis unit did put most of them at risk.
Studies from China and Europe on ESKD patients with COVID-19 were limited to small numbers and single centers. One of the first studies from US from CUMC was limited by less then 100 patients as well. It did show poor outcomes of 59 patients where 31% had died.

A Study from UK did discuss the concerns for an urban dialysis center ( on risk of hospitalizations). Of 1530 patients (median age 66 years; 58.2% men) receiving dialysis, 300 (19.6%) developed COVID-19 infection, creating a large demand for isolated outpatient dialysis and inpatient beds. An analysis that included 1219 patients attending satellite dialysis clinics found that older age was a risk factor for infection. COVID-19 infection was substantially more likely to occur among patients on in-center dialysis compared with those dialyzing at home.
A study from the Bronx in NY also showed poor outcomes for hospitalized ESKD patients. Elevated inflammatory markers were associated with in hospital death.
Another UK study also found a high prevalence of seropositivity in the outpatient dialysis units.

Alberici et al.describe their clinical experience with MHD patients cared for at 4 outpatient dialysis facilities that are part of the Brescia Renal COVID Task Force. In a period of 1 month, viral positivity was detected in 94 of their 643 ESRD HD patients (15%). Important findings in the study were the mild form of symptomatology at presentation, the high rate of overall mortality (29%), and emergence of usual risk factors for mortality and acute respiratory distress syndrome in SARS-CoV-2–positive HD patients. In addition, although certain patients were deemed more stable and were managed in the outpatient facility, 3 of those subsequently died, and a substantial portion had significant worsening of their symptoms.
Goicoechea et al. describe the clinical course and outcomes of 36 patients from 2 dialysis facilities caring for 282 patients that were admitted to a tertiary hospital in Madrid based on positive reverse transcription polymerase chain reaction for SARS-CoV-2. They report a mortality rate of 30.5%, and 33% of their patients required mechanical ventilation. 
At our health system of over 23 hospitals in NY, we decided to compare the outcomes of ESKD patients to non ESKD patients. The data was from 13 hospitals and our
final cohort had 419 (4%) with ESKD and 10,063 (96%) without ESKD.This is the largest study to date.

What did we find:( similar tweetorial by first author Jia Ng)
1. Patients with ESKD were older,
had a greater percentage self-identified as Black, and more comorbid
conditions.
2. Patients with ESKD had a higher
rate of in-hospital death than those without (31.7% vs 25.4%), odds ratio 1.38,
95% confidence interval 1.12 - 1.70). This increase rate remained after
adjusting for demographic and comorbid conditions (adjusted odds ratio 1.37,
1.09 - 1.73).
3. Patients with ESKD had similar
rates of mechanical ventilation as those without ESKD (89 [21.2%] vs 2076
[20.6%]). There was no difference in the odds of mechanical ventilation between
the groups.
4. The odds of length of stay of seven or more days was
higher in the group with compared to the group without ESKD in both the crude
(1.62, 95%CI 1.27 - 2.06) and in the adjusted analysis (1.57, 95% 1.22 - 2.02)
5. We conducted stratified analyses to investigate the risk
factors of death in the subgroups of ESKD and the non-ESKD separately, with the
hypothesis that the risk factors of death and the magnitude of risk factors
would differ between the two groups.
6. For patients without ESKD, the independent risk factors
for in-hospital death increased age, male sex, cardiovascular disease, cancer,
requiring ventilation, requiring vasoactive meds, high blood urea nitrogen, low
albumin, high CRP and high ferritin.
7. The diagnosis of hypertension and use of an ACE inhibitor
or ARB were associated with a lower risk of in-hospital death in the non-ESKD
group.
8. Among patients with ESKD, independent risk factors for
in-hospital death were increased age, requiring ventilation and lymphopenia,
elevated BUN and high serum ferritin. Black race was associated with a
significantly lower risk of death among patients with ESKD.
9. The protective effect of HTN in the non-EKSD group, and
the protective effect of Black race in the ESKD group defy easy explanation. Perhaps APOL1 has some protective cardiac effect?
10. This is a large cohort of hospitalized
patients with #COVID-19 comparing ESKD and non-ESKD in a diverse patient population.
We had prespecified operational definitions for exposures, covariates and
outcomes, as well as rigorous adjudication by two independent reviewers for
ESKD exposure.
11. What limitations do we have?--Despite the larger size
of this study compared to other reports, the ESKD sample may still have been
relatively underpowered to find other statistically significant risk factors in
mortality. Also there was inability to adjust for remdesivir
and dexamethasone. As the evidence of these 2 drugs came after the surge of #COVID-19
cases in our health system, only a small proportion of patients received these
drugs.
12. We had 11 PD patients in our admitted cohort. This was also published in a special report as well. Of 419 hospitalized patients with ESKD, 11 were on chronic PD therapy (2.6%). Among those 11, 3 patients required mechanical ventilation, 2 of whom died. Of the entire cohort, 9 of the 11 patients (82%) were discharged alive. While fever was a common presentation, more than half of our patients also presented with diarrhea. Interestingly, 3 patients were diagnosed with culture-negative peritonitis during their hospitalization. Seven patients reported positive SARS-CoV-2 exposure from a member of their household.
In conclusion, among patients hospitalized with COVID-19,
those with ESKD had a higher rate of in-hospital death compared to those
without ESKD.
Two recent studies also show the outpatient HD infection and admission rates. A study published in AJKD from Canada showed from universal screening, 4.6% were infected.
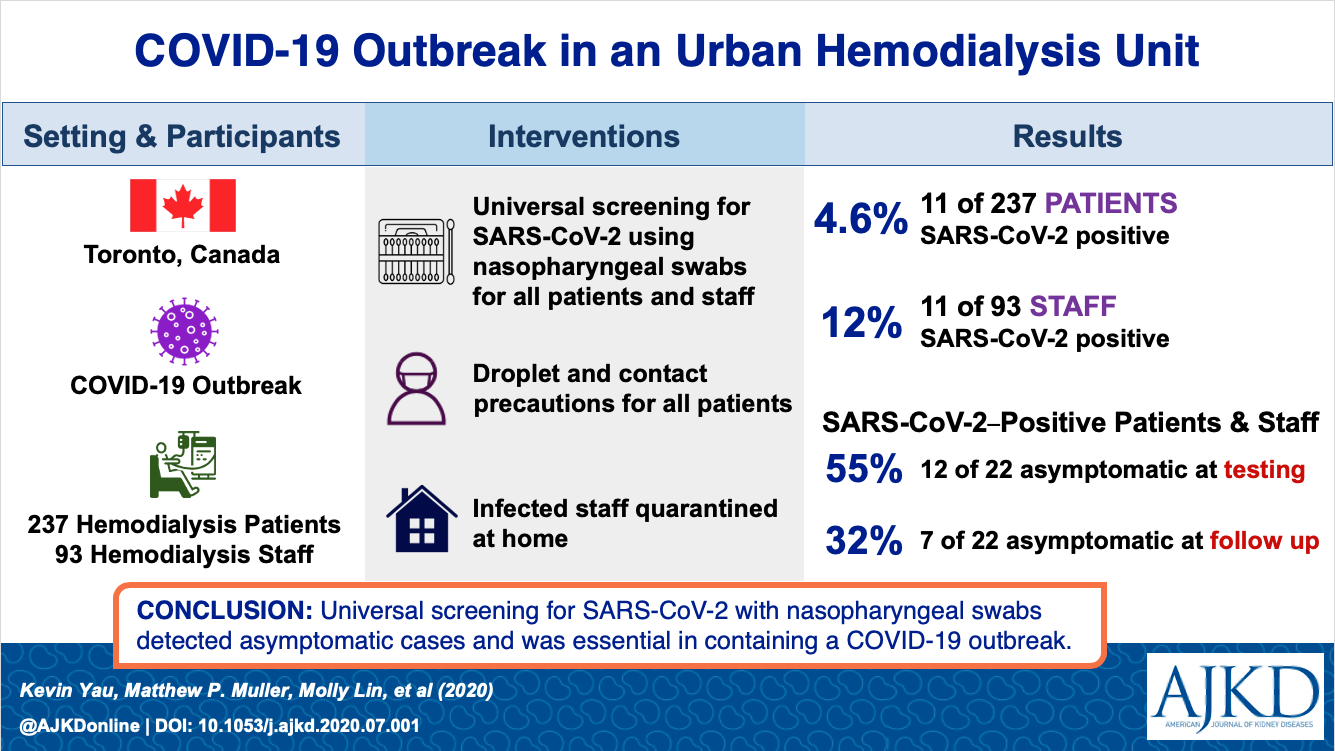
Another French study in KI showed a low incidence of infection of 3.3% in a large >40,000 dialysis patients. Older age, low albumin, and cardiac disease were risk factors for mortality.

Taken together, the results suggest both a need for further
research and the continued need for careful infection control procedures in the
ESKD population at risk for #COVID-19.










