Recently, few of us have noticed we get emails inviting us to submit to " Journal .... nephrology.. and .." The best one I got one was " New England Journal of Nephrology". In addition, there are apparently these emails inviting you to be presenting at conferences that sound so real but for few of us who have been in academia- know that they are a money making scam. What is gained from these conferences?
Series of these conferences are run by OMICS. They are apparently for many different fields in medicine. When I clicked on the Nephrology section for the journals, I got this
https://www.omicsonline.org/nephrology-journals.php ( mostly Non nephrology journals)
When clicked on conferences, you get these
http://www.conferenceseries.com/nephrology-meetings
Now take a look at the locations: Las Vegas, Orlando, Dubai!!
It seems that they are happening for many years. Has anyone gone to these? Based on the NYT article that was recently published, The Federal Trade Commission formally has charged OMICS with deceiving academics and researchers on such publications and fees.
The author in the NYT says nicely at the end and I quote "So it’s not surprising that some academics have chosen to give one another permission to accumulate publication credits on their C.V.’s and spend some of the departmental travel budget on short holidays. Nor is it surprising that some canny operators have now realized that when standards are loose to begin with, there are healthy profits to be made in the gray areas of academe."
Perhaps as a community in Nephrology we need to create a page that lists which of these journals and conferences are FAKE and prevent our colleagues on spending money on these ventures.
Thursday, December 29, 2016
Wednesday, December 14, 2016
Lupus Nephritis classification: Does it help us?
I recently went to a talk by Stephen Korbet on Lupus Nephritis and it got me wondering on if the current way of classification of lupus nephritis works or not?
MPGN- old way of classification was EM based but the recent updated IF based classification is very clinical and aids the clinician in treating the disease better as a root cause is identified.
In lupus, the story starts back in 1970s and eventually leading to the WHO classification and then the updated ISN classification. A recent review published in JASN in 2015 summarizes the history and concerns regarding the classifications. The suggestions to improve are more detailed and pathology related and I am not sure if they will help clinically.
What might help a nephrologist help treat the lupus nephritis patient?
1. Is the lesion Proliferative?- segmental or diffuse- most of us will treat. Only context of not treating will be the IFTA present on the biopsy- so does it matter if its segmental or diffuse? as treatment is either MMF or cyclophosphamide anyway.
2. Is the lesion crescentic? - yes this matters to us-- as most crescentic GN( RPGN) and specifically lupus have been excluded in most trials- so treatment might be leaning towards cytotoxic agents and not standard therapy.
3. Is it a podocytopathy ( would like to include membranous GN in this section)- More and more we are seeing MCD, FSGS with this entity and treatment might be slightly different as some of them respond faster with a steroid based regimen.
4. Is there a second entity with it?- ANCA disease or TMA?- as treatment might then entail pheresis and or a different prognosis.
I think the talk by Korbet hinted towards this but not sure which direction the field will go but it's time that we have a less confusing classification but more meaningful one that helps the nephrologists treat the disease better.
What do others think?
Thursday, November 24, 2016
Topic Discussion: Novel anticoagulants in CKD and ESRD
New
Oral Anticoagulants(NOACs)
|
Renal
clearance of parent drug
|
Dosage
in ESRD
|
GFR
15-29ml/min
|
GFR
30-40ml/min
|
GFR
>-50ml/min
|
Dialyzable
(yes/no)
|
Reversal
agent
|
Dabigatran( Direct thrombin inhibitor)
|
80%
|
Avoid
|
75mg BID
|
150mg BID
|
150mg BID
|
Yes with 10% rebound rate
|
Idarucizumab
Dialysis
|
Rivaroxaban(Factor Xa inhibitor)
|
36%
|
15mg QD
|
15mg QD
|
15mg QD
|
20mg QD
|
No
|
4-factor prothrombin complex
concentrate
Andexanet Alfa |
Apixaban( Factor Xa inhibitor)
|
27%
|
5mg BID
|
2.5mg BID
|
5mg BID
|
5mg BID
|
No
|
4-factor prothrombin complex
concentrate
Andexanet Alfa |
Edoxaban (Factor Xa inhibitor)
|
50%
|
30mg QD
|
30mg QD
|
30mg QD
|
60mg QD
|
No
|
4-factor prothrombin complex
concentrate
Andexanet Alfa |
Patients with atrial fibrillations are being treated with NOACs as they are simple to use, no monitoring and excellent safety profile. They are higher in costs and experience still limited. NOAC use in patients with advanced CKD and on dialysis is substantial and increasing, despite AHA, ACC, and HRS and European Heart Rhythm Association guidelines that endorse warfarin as the anticoagulant of choice when CrCl is <30 ml/min. There are few randomized trial data on NOACs among patients with advanced CKD or on dialysis. Most NOACS are dependent on the kidney for elimination. Since most patients with advanced CKD were excluded in clinical trials, this topic is important.
The above table summarizes what the current data exists on this topic for
use of NOACs in CKD patients with Atrial Fibrillation for prevention of stroke. Bleeding risk is important in patients with
CKD and ESRD due to uremic dysfunction of platelets and use of heparin in
HD. Unfortunately, no patients with CKD
Stage V or ESRD were not allowed in any of the NOAC trials. Apixaban was the most commonly used NOAC in a
recent analysis in advanced CKD patients. Apixaban and Rivaroxaban have renal
eliminations around 30% hence most safe in late stage CKD with lower dosing.
Dabigatran and Edoxaban ar 80% and 50% renal elimination respectively and
should technically avoided in late CKD patients. Dabigatran is the only NOAC removed by dialysis based on studies thus far.
This review in JACC summarizes the latest uptodate information on use of these
agents in CKD and ESRD patients. – A must read!
Labels:
cardiac testing,
cardiology,
CKD and ESRD,
topic discussions
Wednesday, November 9, 2016
TOPIC DISCUSSION: Is THSD7A the paraneoplastic marker for Membranous GN associated with cancer?
Two recent
papers from Germany have now associated the thrombospondin type 1 domain containing
7A(THSD7A) as a target antigen identified in membranous GN in association with cancer. In a large study, the authors screened > 1200
patients for western blot analysis for THSD7A. The incidence was
2.6%. They were mostly women. In this
cohort, the percentage of patients with THSD7A-associated MN and malignant
disease significantly exceeded that of patients with PLA2R-associated MN and malignant disease. In all cohorts, they
identified 40 patients with THSD7A-associated MN, eight of whom developed a
malignancy within a median time of 3 months from diagnosis of MN. In one
patient with THSD7A-associated MN and metastases of an endometrial carcinoma,
immunohistochemistry showed THSD7A expression on the metastatic cells and
within follicular dendritic cells of the metastasis–infiltrated lymph
node.
In a separate
report in NEJM, the same group described a case of gall bladder cancer and
membranous GN. The patient had circulating THSD7A antibodies and THSD7A antigen
positive membranous GN. The primary gall
bladder tumor and lymph nodes also stained for THSD7A on the
immunohistochemical analysis. Following
chemotherapy, the THSD7A antibodies in plasma were no longer detectable and
proteinuria improved as well. In that study, when additional 1009 patients with
membranous were reviewed, 25 had positive THSD7A antibodies. Of the 25, 7 had
malignant tumors.
Patients
with THSD7A-associated MN differ in their clinical characteristics from
patients with PLA2R1-associated MN, and more intensive screening for
the presence of malignancies may be warranted in those with THSD7A-associated
MN.
Labels:
membranous GN,
onco nephrology,
topic discussions
Tuesday, November 8, 2016
Topic Discussion: Serology based treatment of Membranous GN
Gone are the days of a kidney biopsy for Membranous GN… Can
that happen? Given the advent of PLAR2
antibody titers availability clinically, can we embark on a serological based
approach to diagnosing and treatment of PLAR2 associated Membranous GN. Here is a proposal from Glassock, Fervenza, Sethi in JASN( Not evidence based at this point but
pathophysiology and common sense based)
I think figures 4,5,6 summarize the entire paper nicely and
are good flow charts for clinical use.
1.
Start with measurement of PLAR2 levels and
screening for secondary causes.
2.
If PLAR2 is positive and no secondary cause, you
have diagnosed PLAR2 associated membranous GN( perhaps no biopsy necessary—my editorial
comment)
3.
If PLAR2 is negative, a kidney biopsy is
mandatory ( if no contraindications and there should be PLAR2 antigen staining
done on it)
4.
If PLAR2 antigen is positive on the biopsy—it’s
likely a PLAR2 associated membranous GN and perhaps in immunological remission
as PLAR2 antibodies were negative. If
the PLAR2 antigen in kidney is negative,
measurement of THDS7A antibody in serum and it’s antigen staining in the
kidney should be performed. If that is positive, you have diagnosed THDS7A
associated membranous GN and that has a strong association with cancer and
hence aggressive screening for cancer
needs to be done. IF it is PLAR2 antigen
and THDS7A antigen negative but IgG subclass 3 positive, secondary causes need
to be considered as this is secondary membranous GN.
5.
Once diagnosed with PLAR2 + membranous GN, and the titer is in the high range( highest
range in the respective lab), and any level of proteinuria, the titer should be repeated twice a month
and if it continues to rise, start cytotoxic agents. If moderate PLAR2 or low and has nephrotic or non nephrotic syndrome, again follow the
titers and if rising, start treatment. If titers are down trending or proteinuria is
improving, no treatment necessary. There is going to be immunological remission
before the proteinuria and clinical remission
6.
If PLAR2 AB response is rapid and >90%
reduction in <6 months, consider stopping treatment
7.
If PLAR2 AB is 50% in 6 months or no response,
consider changing treatment options
8.
If the response is slow (50-90%) at 6 months,
continue treatment for longer time frame.
Tuesday, October 25, 2016
Topic Discussion: AKI following LVAD
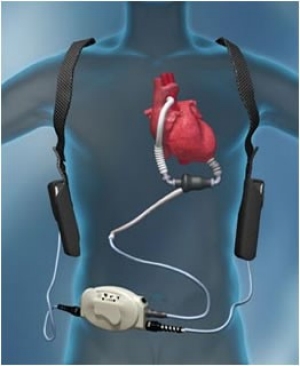 Left ventricular assist devices (LVADs) are used increasingly as a bridge to transplantation or as
destination therapy in end-stage heart failure patients who do not respond to optimal medical
therapy. Many of these patients have end-organ dysfunction, including advanced kidney dysfunction,
before and after LVAD implantation. Kidney dysfunction is a marker of adverse outcomes,
such as increased morbidity and mortality.The incidence of AKI after LVAD implantation varies considerably: between 4 and 38 %.
Left ventricular assist devices (LVADs) are used increasingly as a bridge to transplantation or as
destination therapy in end-stage heart failure patients who do not respond to optimal medical
therapy. Many of these patients have end-organ dysfunction, including advanced kidney dysfunction,
before and after LVAD implantation. Kidney dysfunction is a marker of adverse outcomes,
such as increased morbidity and mortality.The incidence of AKI after LVAD implantation varies considerably: between 4 and 38 %. Risk factors:
INTERMACS score 1 or 2 ( http://content.onlinejacc.org/article.aspx?articleid=1143100)
Kidney <10 cm in size
Older age
ACE-I or ARB therapy immediately prior to surgery
High central venous pressure
Low LV end-diastolic dimensions
Long CPB time
Higher intraop bleeding
Need for re operation
Sepsis
Liver dysfunction
Need for blood transfusions
RV failure
CKD
Factors such as acute blood loss, volume shifts, arrhythmias, and the effect of multiple vasoactive medications influence renal hemodynamics. The sudden change in renal blood flow characteristics due to continuous flow-LVAD support can lead to AKI. Patients with preoperative RV failure and patients with INTERMACS scores of 1 or 2 are at higher risk of AKI. The RV function is of vital importance after LVAD placement since postoperative RV failure and idioventricular arrhythmias have been associated with AKI . An RV dysfunction can result in a reduced LV preload, low LVAD speeds, reduced forward flow, increased arrhythmias, and liver as well as kidney congestion.
It appears that the cause is hemodynamic vs ATN. No study has really defined the mechanism. Given the LVAD devices have pro thrombotic risk , renal vascular thrombosis and or anticoagulation related AKI should be in the differential. As always, AIN from any medication is to be considered. Hemolysis related injury could be leading to pigment nephropathy as well.
https://www.ncbi.nlm.nih.gov/pubmed/25759700
https://www.ncbi.nlm.nih.gov/pubmed/25796403
Wednesday, October 12, 2016
Nephrology Crosswords: Hypertension
Next installment of Nephrology Crosswords appears in Kidney International in the Nov 2016 issue.
http://www.kidney-international.org/article/S0085-2538(16)30260-5/fulltext on HTN
Labels:
Hypertension,
Nephrology Crosswords
Tuesday, October 4, 2016
In the NEWS: Lung Ultrasound and volume assessment in ESRD
Your new device- the lung ultrasound has made its way in Europe. A recent trail in CJASN discusses the value of using lung ultrasound in assessing volume status of a patient. Lung water can be used in a way in clinical practice as it is being used in critical care and cardiology to assess volume in ESRD patients. Few prior studies that have looked at the role of ESRD and lung ultrasound in clinical practice and training of fellows/faculty are here:
This
new study in CJASN looked at >1000 patients pre and post HD via lung
ultrasound simultaneous to standardized lung exams ( crackles ) and peripheral
edema. What the investigators found was
that the lung congestion by crackles, edema or a combination was inferior to
ultrasound B lines in various analysis. Using
the knowledge of B lines might guide us in better managing volume status in our
ESRD patients.
In
the editorial with it in CJASN, Dr. Rich Sherman writes “I believe that lung US will prove to be
of value in improving the care of patients on dialysis . From a practical
standpoint, I hope that this technique can provide us with at least one simple
benefit. If a routine lung US on the previous Friday before dialysis might make
these events less likely, then sign me up!”
I concur with Dr
Sherman! I think Nephrologists should get familiar with the science and
technology and embrace this change to help their patients. Dialysis units (
large and small) should consider carrying US machines to help guide volume exam
and make clinical decisions. It will decrease hospitalizations, less radiation
exposure( X rays) and hopefully less ER visits.
Image courtesy: coreem.net
Labels:
CKD and ESRD,
In The News,
ultrasound
Monday, October 3, 2016
Concept Map: Electrolyte Disorders and anti cancer agents
Most electrolytes disorders are "hypo" that are drug induced from anti cancer agents. Mechanism is mentioned where there is evidence.
The two references are:
https://www.ncbi.nlm.nih.gov/pubmed/26939882
http://www.kireports.org/article/S2468-0249(16)30134-6/pdf
Labels:
concept maps,
electrolytes,
onco nephrology
Friday, September 30, 2016
Topic Discussion: Complement and the Kidney
 The complement system can be attacked to help treat kidney disease. Complement activation contributes to the pathogenesis of acute and chronic kidney disease injury. The aHUS and C3GN story has led us to believe that there might be hope for other potential targets in the complement system for patients with kidney disease.
The complement system can be attacked to help treat kidney disease. Complement activation contributes to the pathogenesis of acute and chronic kidney disease injury. The aHUS and C3GN story has led us to believe that there might be hope for other potential targets in the complement system for patients with kidney disease.A recent mini review in KI summarizes the role of the complement system in kidney disease and where future drugs hold promise. The complement activation is initiated via 3 pathways- classical, alternative and lectin. Full activation leads to the generation of several biologically active fragments, namely C3a, C5a, C3b and C5b-9. Drugs are currently being developed to block the classical pathway, the alternative pathway and the activation at the level of c3,c5 and c5a.
C1 inhibitors, TNT009( anti C1s) affect the classical pathway
Purified factor H, anti Factor D agents, CR2-factor H, affect the alternative pathway
Compstatin and soluble CR1 inhibits at level of C3
Eculizumab and other anti C5 inhibit at level of C5
and CCX168 inhibits at level of C5a
Check out two excellent reviews, one in KI and other in KIR
http://www.kidney-international.org/article/S0085-2538(16)30185-5/fulltext
http://www.kireports.org/article/S2468-0249(16)30031-6/fulltext
Labels:
basic science,
complements,
glomerular diseases
Sunday, September 25, 2016
Targeted therapies and the Kidney
 Novel targeted anti-cancer therapies have resulted in improvement in patient survival compared to standard chemotherapy. Renal toxicities of targeted agents are increasingly being recognized.
Novel targeted anti-cancer therapies have resulted in improvement in patient survival compared to standard chemotherapy. Renal toxicities of targeted agents are increasingly being recognized.
The incidence, severity, and pattern of renal toxicities may vary according to the respective target of the drug. A recent uptodate review by us discusses the adverse renal effects associated with a selection of currently approved targeted cancer therapies, directed to EGFR, HER2, BRAF, MEK, ALK, PD1/PDL1, CTLA-4, and novel agents targeted to VEGF/R and TKIs.
Based on another study and look at the FDA database, electrolyte disorders, renal impairment and hypertension are the most commonly reported events with this agent. Of the novel targeted agents, ipilumumab and cetuximab have the most nephrotoxic events reported.
Novel agents have also been tried in myeloma treatment. Renal effects of these agents are being reported as case reports and parts of clinical trials. A recent review in CJASN summaries the novel toxicities associated with new anti myeloma agents.
The early diagnosis and prompt recognition of these renal adverse events are essential for the general nephrologist taking care of these patients.
Labels:
chemotherapy,
myeloma,
onco nephrology
Tuesday, September 20, 2016
Topic Discussion: Collapsing FSGS and TMA
Endothelial
damage as a missing link… perhaps. Recent
study published in KI tries to link TMA as a cause of collapsing variant of
FSGS or CG. They looked at 53 patients
with renal limited TMA in a native kidney with emphasis on looking for
FSGS. 33 of the 53 had FSGS( mostly 19
being CG, 9 with NOS type, 3 with cellular and rest perihalar and tip
variant).
Some interesting findings:
1.
Prognosis
of TMA with FSGS was worse than TMA alone
2.
Most
of the patients with TMA were from HTN followed by complement disorders, drugs
and other causes. The more diffuse the TMA in the kidney in the 53 patients,
the more likely they would have systemic TMA, higher crt and higher BP
3.
At the time of the renal biopsy, there
was no significant difference between TMA without FSGS, TMA-CG, and TMA
with other FSGS variants with respect to age, sex, and ethnicity. The degree of
renal impairment also did not differ among the 3 groups. Proteinuria was
significantly higher(2.5gm) in cases with FSGS (CG and other FSGS variants) than in
cases without FSGS(1.42gm). Nevertheless, there was no difference of proteinuria
between CG and the “other FSGS” category (2.39 vs 2.72gm)
4.
The frequency of nephrotic syndrome was low in
each group (5.9%, 11.8%, and 7.7% in “no FSGS,” CG, and “other FSGS” groups,
respectively.
This is interesting as FSGS classically presents with significant proteinuria.
5.
TMA associated CG and “classical” CG (i.e., CG related
to ethnicity, viruses, or drugs, or a combination of these) differ on many
points although they are indistinguishable by light microscopy. Classic CG usually is seen in blacks, there they
saw it in whites more. The nephrotic syndrome
is more severe in classic CG compared to TMA associated CG. Third, the authors found
that dysregulation of the immunohistochemical phenotype of podocytes was
less marked in our TMA-CG cases than in “classical” CG: although we observed
podocyte dedifferentiation in one-half of the tested cases, proliferation of
podocytes was not detected. This result is in accordance with the fact that the
degree of podocyte dysregulation is less prominent in the reactive forms of CG.
6.
TMA-CG is associated with attenuated podocyte
changes relative to “classical” CG and may be insufficient to trigger a
full-blown clinical, immunohistochemical, and ultrastructural phenotype.
7.
Perhaps the TMA came first and led to HTN and
ischemia and that leads to CG( hence the less severe proteinuria). Or is one
protecting the other to keep
the VEGF balance as too little VEGF leads to TMA and too much to CG.
8.
Clearly, this is an important association and
finally something that can be seen in practice. Classically this is seen in HTN
as it can lead to both forms of endothelial injury.
9.
Similar concepts have been noticed in post
transplant CG in a prior post
Labels:
FSGS,
glomerular diseases,
TMA,
topic discussions
Tuesday, September 6, 2016
CONSULT ROUNDS: Glomerular disease with Sickle cell disease
From the largest case series of 18 biopsies
The four most common histology in the glomeruli were
FSGS ( 39%)-- any variant--likely due to hypoxia
MPGN(28%)- not sure if this was immuglobulin only or complement only- but likely null IF making omre likely a chronic TMA
TMA( 17%)
Sickle cell glomerulopathy( 17%)
Here is a nice recent review by Karl Nath( editor in chief of JASN)
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4701210/
The four most common histology in the glomeruli were
FSGS ( 39%)-- any variant--likely due to hypoxia
MPGN(28%)- not sure if this was immuglobulin only or complement only- but likely null IF making omre likely a chronic TMA
TMA( 17%)
Sickle cell glomerulopathy( 17%)
Here is a nice recent review by Karl Nath( editor in chief of JASN)
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4701210/
Labels:
Consult Rounds,
glomerular diseases,
sickle cell
Thursday, September 1, 2016
CME on Onconephrology at MD Anderson
Onconephrology Symposium at MD
Anderson, Texax
When: Friday, October 14, 2016, from 8:30 am to 5:00 pm
Where: Onstead Auditorium, 6767
Bertner Ave, Houston, TX 77030
(Discounted conference rates at
nearby Marriott Hotel)
Who: Healthcare professionals
interested in cancer and the kidney
Topics: Nephrotoxicity of
chemotherapy and targeted therapy, AKI in cancer, CKD in stem cell transplant,
erythropoiesis agents, hyponatremia, hypercalcemia, ethics, dialysis and CRRT,
myeloma and more!!
Speakers: Mark Perazella, Sangeeta
Hingorani, Kenar Jhaveri, Ala Abudayyeh, Katy Rezvani, Steven Fishbane,
Mitchell Rosner, Biff Palmer, Jennifer Scherer, Kevin Finkel, Amit Lahoti
Register at http://tinyurl.com/MDA-
Tuesday, August 23, 2016
Targeted therapies and tumor lysis syndrome
Novel targeted therapies are
being approved in clinical trials for many hematologic malignancies. Tumor
lysis syndrome (TLS) is being noticed as a novel side effect of many of these
agents. A recent review
in AJH summarizes the various drugs that have led to TLS as a potential
side effect of targeted therapies.
TLS was most common in drug
trials dealing with patients with acute leukemias, high grade non Hodgkin’s
lymphomas, mantle cell lymphomas, CLL and myeloma. Some of the risk might be
tumor related rather than the drug but below are the drugs that could be
potential TLS promoting.
Incidence of TLS based on
clinical trials for novel targeted therapies
Alvocidib (cyclin dependent
kinase inhibitor) – 42% in poor risk AML patients, 13% in CLL patients
Venetoclax( ABT-199)( small
molecule B cell lymphoma/leukemia 2 inhibitor)- 2.7-8.9% in CLL
Dinaciclib( cyclin dependent
kinase inhibitor)- 15% in patients with AML or ALL and another 15% in CLL
Ibrutinib( Bruton kinase
inhibitor)- 6.7% in CLL patients
Dasatinib( BCR-ABL tyrosine
kinase inhibitors)- 4.2% in patients with ALL
Lenalidomide( immunomodulatory
agent)- 3-4% in CLL patients
Obinutuzumab( anti CD20 agent)-
3-4.8% in CLL patients
Oprozomib( proteasome inhibitor)-
2.4% in various hematologic malignancies
Brentuximab vedotin( anti cd30
antibody)- 1.7% in anaplastic large cell lymphoma patients
Carfilzomib( proteasome inhibitor)-
1% in myeloma patients
Not much found in the two listed below
Idelasib( phosphatidylinositol
3-kinase inhibitor)- No TLS in CLL patients
Ofatumumab( anti CD20 agent)-No
TLS in CLL patients
Wednesday, August 17, 2016
Topic Discussion: Euglycemic Diabetic Ketoacidosis
 The first time the term Euglycemic DKA(eDKA) was mentioned was in 1973- in British Medical Journal in patients who were diabetic but didn't have the full blown hyperglyecmic part. Compared to classic DKA, eDKA presents with mild to moderate hyperglycemia typically <300mg/dl blood glucose levels.
The first time the term Euglycemic DKA(eDKA) was mentioned was in 1973- in British Medical Journal in patients who were diabetic but didn't have the full blown hyperglyecmic part. Compared to classic DKA, eDKA presents with mild to moderate hyperglycemia typically <300mg/dl blood glucose levels. Why is this more important now?
In 2013, many SGLT2 inhibitors got approved for DM management( the glucoretics). The FDA performed a FAERS search of adverse effects with these agents and 73 cases were identified of ketoacidosis linked to SGLT-2 inhibitors. All patients required hospitalization, and 60% had DMII. Blood glucose levels ranged from 90mg/dl - 1300mg/dl( median 211). Timing of onset was around 43 days or starting or dose change of the agent. Majority of the cases also had dehydration, infection or change in insulin doses. No mortality has been reported with this effect. All patients respond quickly with intravenous hydration and insulin once recognized. The FDA did acknowledge that some of the cases occurred in DMI, where it's an off label use. More detail here.
Is it a class effect?
Yes. The initial FDA reporting was done with canagliflozin(invokana). A more recent study found an incidence rate of 0.07% with this agent. In a large study with dapagliflozin( Farxiga), 0.1% of patients got eDKA. Empagliflozin(Jardiance) also has been found to cause eDKA.
What are the risk factors for development of eDKA with SGLT2 inhibitors?
Dehydration
Alcohol use
decrease in insulin use
Infection
Low carbohydrate diet
Reduction in caloric intake
Advance age
Mechanism of action
Ketosis results from
restriction of carbohydrate usage with increased reliance on fat oxidation for
energy production. The pathogenesis of hyperglyemic DKA is well established. Since SGLT2 are glucoretics
as described before, they can lead to volume depletion- like a diuretic and
perhaps leading to a "starvation" like ketoacidosis with normal
glucose levels. SGLT2 induced glycosuria can happen over 24 hours and
this artificial low plasma glucose do not stimulate insulin. In eDKA, insulin deficiency and insulin resistance are
milder; therefore, glucose overproduction and under-utilization are quantitatively
lesser than in DKA. More importantly, renal glucose clearance (i.e., the ratio
of glycosuria to prevailing glycemia) is twice as large with eDKA than with
DKA. Ketoacidosis follows with the same sequence of events in eDKA as in
DKA. Insufficient insulin levels will then decrease glucose
utilization and promote lipolysis and ketogenesis. In addition, these drugs can
increase glucagon levels leading to increase ketone production.
In summary, eDKA is
pathophysiologically similar to DKA except
for the circumstance—SGLT2-induced glycosuria—that “artificially” lowers plasma
glucose levels and predisposes to increased ketogenesis.
Prevention and treatment
Blood and urine monitoring of ketones is essential especially when patients get ill or are experiencing one of the risk factors. Adequate hydration and carbohydrate intake will help and holding the offending agent is indicated. No data exists on a safe time to restart the agent.
Here is a nice review on this topic
http://onlinelibrary.wiley.com/doi/10.1111/jdi.12401/pdf
Labels:
acid base,
endocrine,
metabolic acidosis,
topic discussions
Thursday, August 11, 2016
Detective Nephron: the next case

Check out the next venture of Detective Nephron in the August issue of Kidney News 2016
Labels:
Detective Nephron,
electrolytes
Thursday, August 4, 2016
TOPIC DISCUSSION: New anti retrovirals and the kidney
As HIV becomes a chronic illness, novel agents to combat this virus have been out in the last decade. We are familiar with the renal toxicities of tenofivir for many years. Tenofivir is the cisplatin of the ID world. But what about the new novel agents?
Here is a table that summarizes the new agents and their known or unknown renal toxicities
Here is a table that summarizes the new agents and their known or unknown renal toxicities
|
Drug
name( trade)
|
Mechanism
of Action
|
Renal
effect
|
Excretion
|
Reference
|
|
Rilpivirine(Edurant)
|
Non
nucleoside reverse transcriptase inhibitor
|
Decreases
the secretion of creatinine in the proximal tubule via OCT2 , appear after
1-2 weeks after treatment and can then plateau
|
Renal
|
|
|
Dolutegravir(Tivicay)
|
Integrase
inhibitor
|
Decreases
the secretion of creatinine in the proximal tubule via OCT2, appear 1-2 weeks
after treatment and then plateau
|
Liver
|
|
|
Cobicistat(Tybost)
|
Inhibitor
liver enzymes so other HIV drugs effect gets enhanced
|
Decreases
in secretion of creatinine in the
apical side of proximal tubule as it inhibits MATE 1 transporter, can be as
early as day 7 of start.
|
Liver
|
|
Tenofivir dispoxil fumarate( TDF) is the classic nephrotoxic agent. It actively taken up by the proximal tubular cell via OAT1 and 3 and released in urinary space by secretion of MDRP-2 and 4. There is a novel tenofivir formulation called tenofivir alafenamide fumrate( TAF) which is used in lower dosage combinations and lower level of parent drug is noted. In addition, TAF does not bind to these organic transporters and hence less renal tubular trafficking. Once out in clinical use, we might see less tenofivir related renal toxicities.
A lot of combination agents are being used to combat the virus. The list below from FDA approved AIDS website compiles them with their trade names. The most nephrotoxic ones are the ones that contain TDF as expected or one of the above mentioned agents that block creatinine secretion.
|
abacavir and lamivudine
(abacavir sulfate / lamivudine, ABC / 3TC) |
Epzicom
|
No renal concerns
|
|
abacavir,
dolutegravir, and lamivudine
(abacavir sulfate / dolutegravir sodium / lamivudine, ABC / DTG / 3TC) |
Triumeq
|
Slight increase in creatinine
|
|
abacavir,
lamivudine, and zidovudine
(abacavir sulfate / lamivudine / zidovudine, ABC / 3TC / ZDV) |
Trizivir
|
No renal concerns
|
|
atazanavir and cobicistat
(atazanavir sulfate / cobicistat, ATV / COBI) |
Evotaz
|
Slight increase in creatinine
|
|
darunavir and
cobicistat
(darunavir ethanolate / cobicistat, DRV / COBI) |
Prezcobix
|
Slight increase in creatinine
|
|
efavirenz,
emtricitabine, and tenofovir disoproxil fumarate
(efavirenz / emtricitabine / tenofovir, efavirenz / emtricitabine / tenofovir DF, EFV / FTC / TDF) |
Atripla
|
Contains tenofivir- renal toxicity can be
present
|
|
elvitegravir,
cobicistat, emtricitabine, and tenofovir alafenamide fumarate
(elvitegravir / cobicistat / emtricitabine / tenofovir alafenamide, EVG / COBI / FTC / TAF) |
Genvoya
|
Contains tenofivir
|
|
elvitegravir,
cobicistat, emtricitabine, and tenofovir disoproxil fumarate
(QUAD, EVG / COBI / FTC / TDF) |
Stribild
|
Contains tenofivir
|
|
emtricitabine,
rilpivirine, and tenofovir alafenamide
(emtricitabine / rilpivirine / tenofovir AF, emtricitabine / rilpivirine / tenofovir alafenamide fumarate, emtricitabine / rilpivirine hydrochloride / tenofovir AF, emtricitabine / rilpivirine hydrochloride / tenofovir alafenamide, emtricitabine / rilpivirine hydrochloride / tenofovir alafenamide fumarate, FTC / RPV / TAF) |
Odefsey
|
Tenofvir alafenamide does not bind to the
proximal tubule transporters and is potentially less nephrotoxic
|
|
emtricitabine,
rilpivirine, and tenofovir disoproxil fumarate
(emtricitabine / rilpivirine hydrochloride / tenofovir disoproxil fumarate, emtricitabine / rilpivirine / tenofovir, FTC / RPV / TDF) |
Complera
|
Contains tenofivir- hence renal failure can
occur and rilpivirine can increase crt as well
|
|
emtricitabine
and tenofovir alafenamide
(emtricitabine / tenofovir AF, emtricitabine / tenofovir alafenamide fumarate, FTC / TAF) |
Descovy
|
Novel tenovifir- hence less likely
|
|
emtricitabine
and tenofovir disoproxil fumarate
(emtricitabine / tenofovir, FTC / TDF) |
Truvada
|
Can cause AKI given tenofivir presence
|
|
lamivudine
and zidovudine
(3TC / ZDV) |
Combivir
|
No renal issues
|
|
lopinavir and
ritonavir
(ritonavir-boosted lopinavir, LPV/r, LPV / RTV) |
Kaletra
|
No renal issues
|
Labels:
drug toxicities,
HIV,
infections,
topic discussions
Subscribe to:
Posts (Atom)




