Friday, August 30, 2024
Tuesday, October 31, 2023
Topic Discussion: Hyponatremia with Spironolactone
Hyponatremia from MRAs is a rare phenomenon but we have encountered it clinically. There are times, when we check labs after starting spironolactone for HTN, 3-4 weeks later, the Potassium is slightly up and the Na comes back 130 or 131 mmol/L. What is the data and the mechanism for hyponatremia following spironolactone use? Is this even related.
In another paper,
high doses of furosemide and
spironolactone, or concomitant use of these diuretics, seem to be an important
cause of hyponatremia in HF patients, particularly in combination with advanced
age, diabetes, and alcohol consumption. Diuretic dose reduction may help avoid
hyponatremia and improve clinical status and prognosis in such patients.
Is it possible that a combination of a thiazide and a K+-sparing diuretic such as amiloride and
spironolactone can increase the risk of hyponatremia because of the enhanced
urinary loss of sodium in the cortical distal tubule? Perhaps not the main
mechanism.
What
about the concept of vasopressin
escape? During hyponatremia, the body limits the degree to which serum
sodium concentration falls through a mechanism called "vasopressin
escape". Vasopressin
escape is a process that prevents the continuous decrease in serum sodium
concentration even under conditions of sustained high plasma vasopressin
levels. In a recent basic science study, the abilities of aldosterone synthase
(Cyp11b2) knockout and wild-type mice to escape from vasopressin were compared.
Wild-type mice escaped while the aldosterone synthase knockout mice did not.
Both the water channel aquaporin 2 (AQP2) and the urea transporter UT-A1
protein abundances were higher in aldosterone synthase knockout than in
wild-type mice at the end of the escape period. Vasopressin escape was also
blunted in rats given spironolactone, a mineralocorticoid receptor blocker. The
authors results indicate that aldosterone regulates vasopressin escape through
calcineurin-mediated protein changes in UT-A1 and AQP2.
So is it possible that we are blunting the natural
vasopressin escape when we combine thiazides with MRAs? Do all MRAs do this?-
this is still unclear. Hyponatremia related to MRAs is an understudied area
worth exploring.
Thursday, August 24, 2023
In the News: Urine Na as a marker for diuresis success
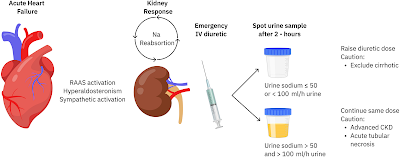
A recent editorial in JAHA discusses the use of urinary sodium (UNa) as a biomarker for monitoring and guiding diuretic therapy in patients with acute heart failure (AHF). Activation of the renin-angiotensin system in heart failure leads to sodium retention, hyperaldosteronism, and increased sympathetic activity, contributing to fluid overload. The authors highlight that assessing diuretic response through traditional methods, such as weight loss and urine volume output, can be inaccurate and logistically challenging. Instead, they propose using UNa measurements from spot urine samples taken 2 hours after diuretic administration as a more dynamic and early indicator of diuretic response.
The European Society of Cardiology (ESC) guidelines recommend using spot UNa analysis to evaluate diuretic treatment response in AHF patients. A low UNa (<50-70 mEq/L) at 2 hours post-diuretic administration is associated with inadequate diuretic response and suggests the need for more intensive diuretic therapy. The paper discusses observational studies and expert opinions that support this approach. However, it also points out limitations, such as the influence of kidney function, concurrent conditions like chronic kidney disease (CKD) and cirrhosis, and the potential loss of UNa's predictive strength after the first day of treatment due to changes in sodium excretion patterns.
The authors present data from studies that endorse the feasibility and efficacy of UNa-guided diuretic therapy in AHF. They discuss the ENACT HF trial, which showed improved natriuresis, diuresis, and shorter hospitalization duration with UNa-guided diuretic treatment. Another ongoing study, PUSH-AHF, aims to provide more definitive results on natriuresis-guided therapy using a stepwise diuretic approach.
The authors acknowledge that UNa assessment alone may not fully capture diuretic response and recommend combining UNa measurements with other indicators of decongestion, such as urine output. They also emphasize the importance of accounting for different patient factors like fluid overload status, kidney function, and the type of diuretics used.
In conclusion, while UNa-guided diuretic therapy appears promising for AHF management.. interesting and simple to do.
Love this figure from the paper
Sunday, March 14, 2021
Topic Discussion: Chloride in Cardio-Renal Syndrome
At recent ASN Kidney Week 2020, Dr. Amir Kazory really gave a great lecture highlighting the importance of an important ion that often is ignored in CHF and Cardio-renal syndrome.
We should perhaps move away from the Na centric view of CHF.
Some interesting points made in his talk and overall what we know.
1. Hyponatremia is a predictor of CHF outcomes. But when we correct the Na, mortality doesn't improve. - classic V2R antagonist EVEREST trial showed no benefit
2. When we give 3% saline as shown by the Yale group recently in JACC, there is significant weight loss in diuretic resistant patients.
3. The Na restriction in diet has limited evidence that it works
Some interesting data on Cl in CHF.
Contemporary advanced CHF cohort suggest that serum chloride levels at admission are independently and inversely associated with mortality in this one study. The prognostic value of serum sodium in CHF was diminished compared with chloride.
Why does this matter?
Two physiological reasons:
1. Low Chloride can stimulate renin release in macula densa
2. Low intracellular chloride can increase TAL NKCC activity and DCT NCC activity
Interestingly, low chloride patients are also diuretic resistant.
It would be fascinating to see if increasing Cl, without Na really has a good effect on diuresis. Azetazolamide trials are ongoing as a potential way to do this. Could SLGT2i be potentially working via this mechanism? It is very possible that Cl is a more important player than Na in CHF and Cardio-renal syndrome. Fascinating!!
Check out this excellent review. ( also for figure source)