In March
2020, as deaths from COVID-19 surged across the world, we orchestrated the
largest nationwide study of critically ill patients with COVID-19 assembled to
date in the United States. This grassroots, unfunded project was made possible
with the help of over 400 collaborators across the US, including research
coordinators, medical students, residents, fellows, and attendings across a
variety of specialties. Together, we gathered detailed, patient-level data from
over 5,000 patients with COVID-19 admitted to ICUs at 68 sites. This was the
start of STOP-COVID (Study
of the Treatment and Outcomes in Critically Ill Patients With COVID-19).
All data
were painstakingly extracted by manual chart review and entered into a
centralized online database. Here is a snapshot of a few of our recent studies.
*In the first
manuscript, we examined risk factors for 28-day mortality among 2215 critically
ill patients. We found that 784 (35.4%) patients died
within 28 days, with wide interhospital variation in both treatments (e.g.,
proning) and outcomes (e.g., death). Factors associated with death included older
age, male sex, morbid obesity, coronary artery disease, cancer, acute organ
dysfunction, and, notably, admission to a hospital with fewer ICU beds. Admission
to a hospital with <50 versus ≥100 ICU beds associated with a >3-fold
increased risk of death in multivariable analyses. Results are published in JAMA
Internal Medicine

*We utilized
a ‘target trial emulation’ approach to test whether early use of tocilizumab
decreases mortality in critically ill patients with COVID-19. Of 3924 patients
included in our analysis, 433 (11%) were treated with tocilizumab in the first
2 days of ICU admission, and these patients had a 30% lower risk of death
compared with those not treated with tocilizumab. The beneficial effect of
tocilizumab on survival was consistent across categories of age, sex, and
illness severity. Notably, we found that patients with a more rapid disease
trajectory, defined as three days or fewer from symptom onset to ICU admission,
appeared to benefit from tocilizumab to a greater extent than patients with a
slower disease trajectory(60% lower risk of
death). Results are published in
JAMA
Internal Medicine with an accompanying editorial.

*We
studied risk factors for acute kidney injury treated with renal replacement
therapy (AKI-RRT) in 3099 patients. We identified several patient-level
risk factors for AKI-RRT, including chronic kidney disease, male sex, non-White
race, and higher D-dimer.
Among patients who survived to hospital discharge, one in three remained RRT-dependent
at discharge, and one in six remained RRT dependent 60 days after ICU admission.
Results are
published in JASN 
*We investigated the incidence,
risk factors, and outcomes associated with in-hospital cardiac arrest and CPR
in 5019 patients. We found that 14% of patients had in-hospital cardiac arrest,
of whom 57% received CPR. Patients who had in-hospital cardiac arrest were
older, had more comorbidities, and were more likely to be admitted to a
hospital with a smaller number of ICU beds compared with those who did not have
in-hospital cardiac arrest. Cardiac arrest was associated with poor survival,
with only 12% surviving to hospital discharge, and even fewer (only 7%)
surviving to hospital discharge with no more than mildly impaired neurologic
function. Results are published in BMJ

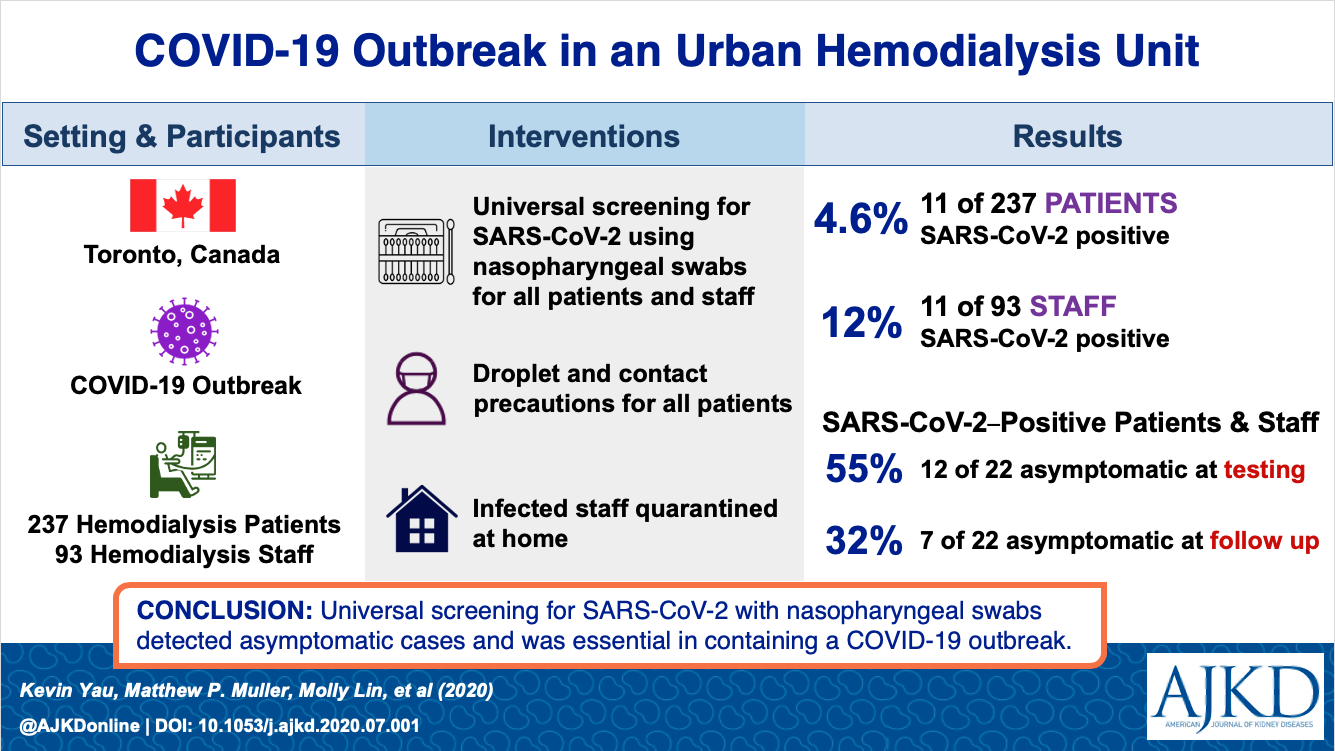
*We examined
the clinical course of critically ill patients with COVID-19 with and without
pre-existing kidney disease. Dialysis patients had a shorter time from symptom
onset to ICU admission compared with other groups, and were more likely to
present with altered mental status on admission. Half the patients with CKD
died within 28 days of ICU admission versus 35% of patients without CKD, with
dialysis patients having the highest risk of death. Results are published in AJKD.

*In a propensity score matched
analysis, we examined the association between solid organ transplant (SOT)
status with death and other clinical outcomes. Receipt of mechanical ventilation,
development of acute respiratory distress syndrome, and receipt of vasopressors
were similar between SOT recipients and non-recipients, as was the risk of
28-day mortality. Results are published in AJT.
Data
collected by the STOP-COVID collaborators has provided valuable insight into the risk factors, outcomes, and treatment strategies for critically ill patients with COVID-19. This is just the
beginning… more to come as we analyze more data.
Shruti Gupta, MD, MPH
David E Leaf, MD, MMsc
---------------------------------------------------------
( Full list of collaborators obtained from JAMA Internal Medicine website)
The Study of the Treatment and Outcomes in Critically Ill Patients With COVID-19 (STOP-COVID) investigators include the following: Carl P. Walther (site principal investigator [PI]) and Samaya J. Anumudu (Baylor College of Medicine); Justin Arunthamakun (site PI), Kathleen F. Kopecky, Gregory P. Milligan, Peter A. McCullough, and Thuy-Duyen Nguyen, (Baylor University Medical Center); Shahzad Shaefi (site PI), Megan L. Krajewski, Sidharth Shankar, Ameeka Pannu, and Juan D. Valencia (Beth Israel Deaconess Medical Center); Sushrut S. Waikar (site PI) and Zoe A. Kibbelaar (Boston Medical Center); Ambarish M. Athavale (site PI), Peter Hart, Shristi Upadhyay, and Ishaan Vohra (Cook County Health); Adam Green (site PI), Jean-Sebastien Rachoin, Christa A. Schorr, and Lisa Shea (Cooper University Health Care); Daniel L. Edmonston (site PI) and Christopher L. Mosher (Duke University Medical Center); Alexandre M. Shehata (site PI), Zaza Cohen, Valerie Allusson, Gabriela Bambrick-Santoyo, Noor ul aain Bhatti, Bijal Mehta, and Aquino Williams (Hackensack Meridian Health Mountainside Medical Center); Samantha K. Brenner (site PI), Patricia Walters, Ronaldo C. Go, and Keith M. Rose (Hackensack Meridian Health Hackensack University Medical Center); Miguel A. Hernán (Harvard T.H. Chan School of Public Health); Rebecca Lisk, Amy M. Zhou, and Ethan C. Kim (Harvard University); Lili Chan (site PI), Kusum S. Mathews (site PI), Steven G. Coca, Deena R. Altman, Aparna Saha, Howard Soh, Huei Hsun Wen, Sonali Bose, Emily A. Leven, Jing G. Wang, Gohar Mosoyan, Girish N. Nadkarni, Pattharawin Pattharanitima, and Emily J. Gallagher (Icahn School of Medicine at Mount Sinai); Allon N. Friedman (site PI), John Guirguis, Rajat Kapoor, Christopher Meshberger, and Katherine J. Kelly (Indiana University School of Medicine/Indiana University Health); Chirag R. Parikh (site PI), Brian T. Garibaldi, Celia P. Corona-Villalobos, Yumeng Wen, Steven Menez, Rubab F. Malik, Carmen Elena Cervantes, and Samir C. Gautam (Johns Hopkins Hospital); Mary C. Mallappallil (site PI), Jie Ouyang, Sabu John, Ernie Yap, Yohannes Melaku, Ibrahim Mohamed, Siddhartha Bajracharya, Isha Puri, Mariah Thaxton, Jyotsna Bhattacharya, John Wagner, and Leon Boudourakis (Kings County Hospital Center); H. Bryant Nguyen (site PI) and Afshin Ahoubim (Loma Linda University); Kianoush Kashani (site PI) and Shahrzad Tehranian (Mayo Clinic, Rochester); Leslie F. Thomas (site PI) and Dheeraj Reddy Sirganagari (Mayo Clinic, Arizona); Pramod K. Guru (site PI) (Mayo Clinic, Florida); Yan Zhou (site PI), Paul A. Bergl, Jesus Rodriguez, Jatan A. Shah, and Mrigank S. Gupta (Medical College of Wisconsin); Princy N. Kumar (site PI), Deepa G. Lazarous, and Seble G. Kassaye (MedStar Georgetown University Hospital); Michal L. Melamed (site PI), Tanya S. Johns, Ryan Mocerino, Kalyan Prudhvi, Denzel Zhu, Rebecca V. Levy, Yorg Azzi, Molly Fisher, Milagros Yunes, Kaltrina Sedaliu, Ladan Golestaneh, Maureen Brogan, Neelja Kumar, Michael Chang, and Jyotsana Thakkar (Montefiore Medical Center/Albert Einstein College of Medicine); Ritesh Raichoudhury (site PI), Akshay Athreya, and Mohamed Farag (New York-Presbyterian Queens Hospital); Edward J. Schenck (site PI), Soo Jung Cho, Maria Plataki, Sergio L. Alvarez-Mulett, Luis G. Gomez-Escobar, Di Pan, Stefi Lee, Jamuna Krishnan, and William Whalen (New York-Presbyterian/Weill Cornell Medical Center); David M. Charytan (site PI), Ashley Macina, Sobaata Chaudhry, Benjamin Wu, and Frank Modersitzki (New York University Langone Hospital); Anand Srivastava (site PI), Alexander S. Leidner, Carlos Martinez, Jacqueline M. Kruser, Richard G. Wunderink, and Alexander J. Hodakowski (Northwestern Memorial Hospital, Northwestern University Feinberg School of Medicine); Juan Carlos Q. Velez (site PI), Eboni G. Price-Haywood, Luis A. Matute-Trochez, Anna E. Hasty, and Muner M. B. Mohamed (Ochsner Medical Center); Rupali S. Avasare (site PI) and David Zonies (site PI) (Oregon Health and Science University Hospital); David E. Leaf (site PI), Shruti Gupta (site PI), Meghan E. Sise, Erik T. Newman, Samah Abu Omar, Kapil K. Pokharel, Shreyak Sharma, Harkarandeep Singh, Simon Correa, Tanveer Shaukat, Omer Kamal, Wei Wang, Heather Yang, Jeffery O. Boateng, Meghan Lee, Ian A. Strohbehn, Jiahua Li, and Ariel L. Mueller (Partners Healthcare, Brigham and Women’s Hospital, Brigham and Women’s Faulkner Hospital, Massachusetts General Hospital, and Newton Wellesley Hospital); Roberta E. Redfern (site PI), Nicholas S. Cairl, Gabriel Naimy, Abeer Abu-Saif, Danyell Hall, and Laura Bickley (ProMedica Health System); Chris Rowan (site PI) and Farah Madhani-Lovely (site PI) (Renown Health); Vasil Peev (site PI), Jochen Reiser, John J. Byun, Andrew Vissing, Esha M. Kapania, Zoe Post, Nilam P. Patel, and Joy-Marie Hermes (Rush University Medical Center); Anne K. Sutherland (site PI), Amee Patrawalla, Diana G. Finkel, Barbara A. Danek, Sowminya Arikapudi, Jeffrey M. Paer, Peter Cangialosi, and Mark Liotta (Rutgers/New Jersey Medical School); Jared Radbel (site PI), Sonika Puri, Jag Sunderram, Matthew T. Scharf, Ayesha Ahmed, Ilya Berim, and Jayanth S. Vatson (Rutgers/Robert Wood Johnson Medical School); Shuchi Anand (site PI), Joseph E. Levitt, and Pablo Garcia (Stanford Healthcare, Stanford University School of Medicine); Suzanne M. Boyle (site PI), Rui Song, and Ali Arif (Temple University Hospital); Jingjing Zhang (site PI), Sang Hoon Woo, Xiaoying Deng, Goni Katz-Greenberg, and Katharine Senter (Thomas Jefferson Health); Moh’d A. Sharshir (site PI) and Vadym V. Rusnak (Tulane Medical Center); Muhammad Imran Ali, Terri Peters, and Kathy Hughes (United Health Services Hospitals); Anip Bansal (site PI), Amber S. Podoll, Michel Chonchol, Sunita Sharma, and Ellen L. Burnham (University of Colorado Anschutz Medical Campus); Arash Rashidi (site PI) and Rana Hejal (University Hospitals Cleveland Medical Center); Eric Judd (site PI), Laura Latta, and Ashita Tolwani (University of Alabama-Birmingham Hospital); Timothy E. Albertson (site PI) and Jason Y. Adams (University of California, Davis, Medical Center); Steven Y. Chang (site PI) and Rebecca M. Beutler (Ronald Reagan-UCLA [University of California, Los Angeles] Medical Center); Carl E. Schulze (Santa Monica-UCLA Medical Center); Etienne Macedo (site PI) and Harin Rhee (University of California, San Diego, Medical Center); Kathleen D. Liu (site PI) and Vasantha K. Jotwani (University of California, San Francisco, Medical Center); Jay L. Koyner (site PI) (University of Chicago Medical Center); Chintan V. Shah (site PI) (University of Florida Health–Gainesville); Vishal Jaikaransingh (site PI) (University of Florida Health–Jacksonville); Stephanie M. Toth-Manikowski (site PI), Min J. Joo (site PI), and James P. Lash (University of Illinois Hospital and Health Sciences System); Javier A. Neyra (site PI) and Nourhan Chaaban (University of Kentucky Medical Center); Rajany Dy (site PI), Alfredo Iardino, Elizabeth H. Au, and Jill H. Sharma (University Medical Center of Southern Nevada); Marie Anne Sosa (site PI), Sabrina Taldone, Gabriel Contreras, David De La Zerda, Alessia Fornoni, and Hayley B. Gershengorn (University of Miami Health System); Salim S. Hayek (site PI), Pennelope Blakely, Hanna Berlin, Tariq U. Azam, Husam Shadid, Michael Pan, Patrick O’Hayer, Chelsea Meloche, Rafey Feroze, Kishan J. Padalia, Abbas Bitar, Jeff Leya, John P. Donnelly, and Andrew J. Admon (University of Michigan); Jennifer E. Flythe (site PI), Matthew J. Tugman, and Emily H. Chang (University of North Carolina School of Medicine); Brent R. Brown (site PI) (University of Oklahoma Health Sciences Center); Amanda K. Leonberg-Yoo (site PI), Ryan C. Spiardi, Todd A. Miano, Meaghan S. Roche, and Charles R. Vasquez (University of Pennsylvania Health System); Amar D. Bansal (site PI), Natalie C. Ernecoff, Sanjana Kapoor, Siddharth Verma, and Huiwen Chen (University of Pittsburgh Medical Center); Csaba P. Kovesdy (site PI), Miklos Z. Molnar (site PI), and Ambreen Azhar (University of Tennessee Health Science Center and Memphis Veterans Affairs Medical Center/Methodist University Hospital); S. Susan Hedayati (site PI), Mridula V. Nadamuni, Shani Shastri, and Duwayne L. Willett (The University of Texas Southwestern Medical Center and Parkland Health and Hospital System); Samuel A. P. Short (University of Vermont Larner College of Medicine); Amanda D. Renaghan (site PI) and Kyle B. Enfield (University of Virginia Health System); Pavan K. Bhatraju (site PI) and A. Bilal Malik (University of Washington Medical Center); Matthew W. Semler (Vanderbilt University Medical Center); Anitha Vijayan (site PI), Christina Mariyam Joy, Tingting Li, Seth Goldberg, and Patricia F. Kao (Washington University in St. Louis/Barnes Jewish Hospital); Greg L. Schumaker (site PI) (Wellforce Health System, Lowell General Hospital); Nitender Goyal (site PI), Anthony J. Faugno, Greg L. Schumaker, Caroline M. Hsu, Asma Tariq, Leah Meyer, Ravi K. Kshirsagar, Aju Jose, and Daniel E. Weiner (Wellforce Health System, Tufts Medical Center); Marta Christov (site PI), Jennifer Griffiths, Sanjeev Gupta, and Aromma Kapoor (Westchester Medical Center); and Perry Wilson (site PI), Tanima Arora, and Ugochukwu Ugwuowo (Yale School of Medicine).