Glomerular disease review in a nutshell- check out the latest offering from AJKD Core Topics review
http://www.ajkd.org/article/S0272-6386(11)01071-7/fulltext
Showing posts with label podocytes. Show all posts
Showing posts with label podocytes. Show all posts
Thursday, October 13, 2011
Sunday, September 18, 2011
Topic Discussion: Cathepsins and the Kidney
 Investigators are getting more and more interested in Cathepsins
and their role in health and disease. Atherosclerosis and diabetes are
closely associated and both involve extensive degradation of the aortic elastin.
Cathepsins are lysosomal cysteine proteases mainly of three types S, L and B.
There are some data on cathepsins involvement in inflammation and
mortality. Recently, in the Sept 2011 issue of JAMA, higher cathepsin S levels
were associated with increased mortality risk in elderly. Studies have
confirmed that elevated levels of cathepsin S has been associated with
worsening diabetes, obesity and atherosclerosis in mice and human models. And
guess what:- the activity of cathepsin S is regulated at the cellular level by
and endogenous inhibitor none other than our friend- cystatin C.
Investigators are getting more and more interested in Cathepsins
and their role in health and disease. Atherosclerosis and diabetes are
closely associated and both involve extensive degradation of the aortic elastin.
Cathepsins are lysosomal cysteine proteases mainly of three types S, L and B.
There are some data on cathepsins involvement in inflammation and
mortality. Recently, in the Sept 2011 issue of JAMA, higher cathepsin S levels
were associated with increased mortality risk in elderly. Studies have
confirmed that elevated levels of cathepsin S has been associated with
worsening diabetes, obesity and atherosclerosis in mice and human models. And
guess what:- the activity of cathepsin S is regulated at the cellular level by
and endogenous inhibitor none other than our friend- cystatin C.
What about the role of cathepsins in renal diseases. The table
below from a recent JCI journal describes the different types of cathepsins and
what their roles are in many diseases.
There is some data that cathepsin L might be involved in
proteinuric renal disease.
Podocytes are unique cells with a complex cellular organization,
consisting of a cell body, major processes, and foot processes . Cell biologic
and mouse genetic studies revealed that many proteins regulate the plasticity
of the podocyte actin cytoskeleton. The onset of proteinuria, induced by either
LPS or puromycin aminonucleoside is associated with the induction of cathepsin
L expression and activity in podocytes. There is an emerging concept that the
onset of proteinuria represents a migratory event in podocyte foot processes
that is associated by the activation of cathepsin L. Podocyte cathepsin L
expression is increased in a variety of human proteinuric kidney diseases,
ranging from minimal change disease (MCD) to diabetic nephropathy. Cathepsin
L–mediated proteolysis plays a critical role in the development of various
forms of proteinuria. Cathepsin L–mediated degradation of dynamin leads to proteinuria in
mice. The notion that dynamin is required for proper
podocyte structure and function is further supported by the observation that
overexpression of dominant-negative dynamin leads to a loss of podocyte stress
fibers in vitro and development of proteinuria in mice.
(image source:- http://www.jci.org/articles/view/42918)
So basically, cathepsins are becoming more and more important players in renal and other non renal diseases. What is interesting is that cystatin C, which is being billed as a renal function marker is an endogenous inhibitor of cathepsin S and an important regulator. So if someone is in renal failure, there is increased cystatin C, there will be increased inhibition of cathepsin S. Either that will increase the levels ( substrate) or decrease it base on how it binds. Hmm.. is it really cystatin C or is it cathepsin S or other cathepsins. Something to ponder and watch out for more work on this field.
Ref:
http://www.jci.org/articles/view/42918 ( currently freely available)
Friday, September 16, 2011
Consult Rounds: Membranous Post Kidney Transplantation
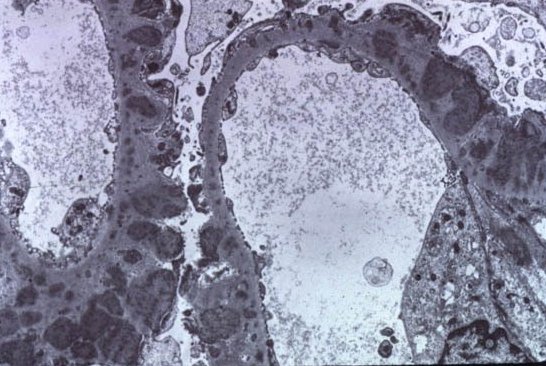 Recurrence of Primary Glomerular Disease is the 3rd most common cause of graft failure.
Recurrence of Primary Glomerular Disease is the 3rd most common cause of graft failure.Membranous GN has a Recurrence post transplant rate of : 30-40% , Usually diagnosed between 2nd-3rd year post transplant. The graft survival is not different than that of patients with other renal diseases. One major study in NDT in 2010 is the only one that really looked at post transplant membranous GN and what happens to those patients. They had 12 patients with recurrence of Membranous. In th 118 month follow up, patient survival was 96% and graft survival was around 40% when compared to controls. Recurrence led to graft loss in 6 patients, all were DDRTx and in 55 months. Recurrence was more common in females. The ones with higher proteinuria did worse. This showed that they did similar patterns as regular Membranous GN.
Another study done recently in 2010 showed that recurrence might be lower compared to previously thought due to being on strong anti rejection agents. The incidence of recurrent iMN was 44%, and recurrences occurred at a median time of 13.6 months after transplantation. Two patterns of recurrence were identified: Early and late. No predictors of recurrence or disease progression could be identified. Anti CD20 agents might be useful in treating this post recurrence.
So really, no good overall data out there. Here are a list of references.
Post by Hitesh Patni, MD
Dr Patni is a renal fellow at the Hofstra NSLIJ School of Medicine
Ref:
http://www.ncbi.nlm.nih.gov/pubmed/12110738
http://www.ncbi.nlm.nih.gov/pubmed/21030574
http://www.ncbi.nlm.nih.gov/pubmed/20466669
http://www.ncbi.nlm.nih.gov/pubmed/20185599
Monday, August 22, 2011
TOPIC DISCUSSION: Retinal-Renal Diseases
 Retinal abnormalities in many inherited renal diseases is common. From CHARGE syndrome, Tuberous Sclerosis, Alports syndrome, LCAT deficiency, to Fabry's disease, VHL Syndrome and Amyloidosis are many diseases that this is noted.
Retinal abnormalities in many inherited renal diseases is common. From CHARGE syndrome, Tuberous Sclerosis, Alports syndrome, LCAT deficiency, to Fabry's disease, VHL Syndrome and Amyloidosis are many diseases that this is noted.Why is that is the question?
The 4 main reasons are:
1. Kidney and Retina develop at the same embryonic stages
2. The Glomerular filtration barrier is very similar to the design of the retinochoroidal junction.
3. Both glomurelus and chorioretina are large capillary beds
4. Both Podocytes( renal epithelial cells) and Retinal epithelial cells are similar in function and depend on cilia for their functions
A nice review is available on this topic in recent issue of JASN August 2011( has a detailed review of all the diseases and mechanisms)
Ref:
http://www.ncbi.nlm.nih.gov/pubmed/21372206
http://www.ncbi.nlm.nih.gov/pubmed/9457747
http://www.ncbi.nlm.nih.gov/pubmed/9227202
Sunday, July 24, 2011
Subscribe to:
Posts (Atom)
