Tuesday, December 31, 2019
Concept map: Immune check point inhibitors and the Kidney
This is the summary of the renal effects of ICI therapy on the kidney ( as of Dec 2019). This might change as we learn more and more about these agents.
Labels:
concept maps,
immunology,
onco nephrology
Thursday, December 19, 2019
Coming in May of 2020- A glomerular disease conference at Northwell
An
Update on Glomerular Diseases, 2020
#northwellGN2020
Saturday, May 2, 2020
7:30am to 6:00pm
North Shore
University Hospital
300 Community
Drive
Manhasset,
New York 11030
@hofstrakidney
Registration information to follow in few weeks
|
||||
|
||||
8:00AM Introduction Kenar D. Jhaveri, MD
8:15AM Membranous
Nephropathy in the PLA2R era Richard
Glassock, MD,
9:00 AM Thrombotic Microangiopathies, A novel
approach, Vanesa Bijol, MD
9:45 AM ANCA and Anti GBM disease in 2020- some old and
some new, Duruvu Geetha, MBBS
10:15AM Break
and exhibits
10:30 AM SGLT-2 inhibitors, diabetic nephropathy and
beyond!! Nupur N Uppal, MD
11:10 AM Treating the right clone- Paraproteinemias Kenar D. Jhaveri, MD
12:00 PM Lunch
and exhibits
1:00 PM MPGN, C3GN..a paradigm shift. Shikha Wadhwani, MD,MS
1:40 PM Drugs, Chemo, Toxins—and the glomeruli. Hitesh H
Shah, MD
2:15 PM IgA Nephropathy- treat or not to treat. Gerald Appel, MD
3:00 PM Podocytopathies, Clinical approach and treatment. Purva Sharma, MD
3:40 PM Treatment of Refractory Lupus Nephritis, Brad H. Rovin, MD
4:15 PM Break
and Exhibit
4:30 PM Did you find my gene for the glomerular
disease??. Barry I. Freedman, MD
5:00PM Case Studies in Glomerular diseases, Vanesa Bijol, MD Purva Sharma ,MD and Kenar D.
Jhaveri,MD
Labels:
conference,
education,
glomerular diseases
Saturday, December 14, 2019
Tuesday, November 26, 2019
Concept Map: Thiazide induced hyponatremias(TAH)
We see this form of hyponatremia in several cases, but recently there has been some newer findings on the mechanisms of TAH(*). In one study published in JCI in 2017, Ware et al showed that there is a subset of patients with a genetic baseline( SLCO2A1 mutation) decrease in prostaglandin(PGE) transport activity which then becomes a risk factor for TAH. So these patients have increased urinary PGE2 and low AVP levels leading to a pure "nephrogenic" cause of tubular water absorption and dilution hyponatremia. PGE2 is critical in insertion and removal of AQP2 channels in the apical membrane. Increased PGE2 signaling leads to insertion of AQP2 channels into membrane and increase water absorption in an ADH independent manner. This is fascinating. Perhaps then mechanism in NSAIDS as well?
Check out this amazing review in AJKD on this topic.
Labels:
concept maps,
diuretics,
hyponatremia,
natremias
Friday, November 22, 2019
Topic Discussion: Zytiga (Abiraterone) induced hypernatremia, and HTN
Zytiga (Abiraterone)
is a hormonal chemotherapy agent used to treat prostate cancer. It selectively and irreversibly inhibits
CYP17 (17 alpha-hydroxylase/C17,20-lyase), an enzyme required for androgen
biosynthesis which is expressed in testicular, adrenal, and prostatic tumor
tissues. Inhibits the formation of the testosterone precursors dehydroepiandrosterone
(DHEA) and androstenedione.
Interestingly. it has
a high rate of hypernatremia as a known renal complication. In several studies,
hypernatremia (33%), hypokalemia (17% to 30%) were reported as known
complications. Why? It is postulated that it can increase mineralocorticoids due
to CYP17 inhibition may result in hypertension, hypokalemia, and fluid
retention (including grade 3 and 4 events) and perhaps some component of
hypernatremia as well- almost like a Cushing's state. Per package insert, concomitant administration
with corticosteroids reduces the incidence and severity of these adverse events.
In the LATITUDE trial, which
used prednisone 5 mg daily in combination with 1000 mg abiraterone acetate
daily, grades 3-4 hypokalemia were detected in 10% of patients on the zytiga arm and 1% of patients on the placebo arm, grades 3-4 hypertension were
observed in 20% of patients on the zytiga arm and 10% of patients on the
placebo arm. Grades 3-4 fluid retention occurred in 1% of patients each arm.
It is
recommended that patients get monitored for hypertension, hypokalemia, and
fluid retention at least once a month. Treatment of hypertension is recommended, choice
of drug is not defined.
This is an
interesting toxicity that as nephrologist seeing prostate cancer with CKD and
perhaps new onset hypertension, hypokalemia or hypernatremia should consider in the
differential diagnosis.
Labels:
electrolytes,
Hypertension,
natremia,
onco nephrology,
topic discussions,
zytiga
Friday, November 15, 2019
In the News: Selinexor induced hyponatremia
A new drug just got approved for treatment for myeloma. It is called selinexor. The correct localization of molecules between nucleus and cytoplasm is fundamental for cellular homeostasis and is controlled by a bidirectional transport system. Exportin 1 (XPO1) regulates the passage of numerous cancer-related proteins. The development of a novel class of antitumor agents, known as selective inhibitors of nuclear export (SINEs) have shown good results in studies and clinical trials in multiple myeloma, non-Hodgkin lymphomas, lymphoblastic leukemia, and acute and chronic myeloid leukemia, sarcomas, and gastric cancer. Selinexor is one of the first to be approved in this class of drugs. In a recent NEJM trial published this year, Chari et al showed that oral selinexor- dexamethasone worked well for triple class refractory multiple myeloma(MM). We wrote a letter back to the authors published in NEJM few weeks later noticing that one of the most common grade 3 or 4 adverse event was hyponatremia(<130mmol/l) ( 22%). In reviewing the prior studies( table below), this is a class effect of selinexor as other trials with the use of this agent had similar rates of hyponatremia ranging from 7%-26%.
Table: Summary of major trials that led to Grade 3,4 hyponatremia
Phase trial
|
Incidence of hyponatremia
|
Intervention
|
Dose modifications
|
Reference
|
Phase 1 in MM
|
25%(40mg/m2),
47% (60mg/m2)
|
Not reported
|
Not reported
Resolved in most cases
| |
Phase 2 in MM
|
22%(80mg)
|
6% got salt tablets,
Dose reduction
|
Yes, reduced
Resolved in most cases
| |
Phase 1 in solid tumors
|
13%
|
Not mentioned
|
Resolved in most cases
| |
Phase 1 in sarcomas
|
7%
|
Not mentioned
|
Resolved in most cases
| |
Phase 1 in Non Hodgkin lymphomas
|
10%
|
Not mentioned
|
Resolved
|
The rates of hyponatremia are higher in the MM studies compared to solid tumor studies. No workup or cause was found in many of the studies. Another recent study in AML ( phase 1) has close to 70% incidence of hyponatremia. Likely this could be related to the GI effects such as severe nausea leading to an ADH release causing hyponatremia or could this be a direct effect of the mechanism of this agent. Could this drug effect the AQP channels or V2 receptor- not sure as mechanism has not been worked out. A serum osmolarity testing along with urine studies can answer this question. As the drug enters clinical practice, It is very possible that we shall see an even increased incidence given other confounders patients might be on such as thiazides, and or increased free water intake. Involvement of nephrology consultation in the trials ongoing might be essential to investigate the mechanism of this toxicity. Serum and urine studies would help in assessment of the cause and pathophysiology of the hyponatremia. This will then allow for preventive strategies in further trials and clinical practice. Once out in the real world, it will be more important as lot of our patients could be on thiazides, SSRi and drinking a lot of water and then are given this agent. While most cases the hyponatremia might be asymptomatic, subtle symptoms and appropriate early management can prevent seizures and complications of hyponatremia.
As nephrologists, we need to be aware of this drug as we usually see myeloma patients.
Labels:
drug toxicities,
hyponatremia,
In The News,
onco nephrology
Wednesday, November 13, 2019
Topic Discussion: Interferons and kidney disease
Interferons usually have been linked with
kidney damage with forms of podocytopathies. CJASN paper from 2010 from CUMC described
these lesions. Collapsing FSGS may
occur after treatment with IFN-alpha, -beta, or -gamma and is typically
accompanied by the ultrastructural finding of endothelial tubuloreticular
inclusions.
Following
that series of 11 patients showing Collapsing GN, few cases reports were published
in 2016 showing FSGS as well. Another large series by Markowitz et al in 2015
of 32 patients also
showed podocytopathies but this time also MCD and FSGS along with
collapsing GN. The MCD patients had complete and partial remission but the FSGS
and collapsing GN had <50% complete or partial remissions.
But the most
common lesion that is hidden in the heme literature is TMA but many being renal
limited. There are now
over 80 cases described of TMA , AKI and HTN related to interferons. Outcome
analysis revealed complete remission in 27 (40%), persistent chronic kidney
disease (CKD) in 28 (42%) and fatality in 12 patients (18%). (10) Treatment
with corticosteroids, plasma exchange and rituximab resulted in durable
responses.
In an elegant
experiment by a group published in 2016 in Blood showed that type 1 interferon
can induced TMA. They showed that the clinical phenotype of cases referred to a
national center is uniformly consistent with a direct dose-dependent
drug-induced TMA with interferon. They then showed that dose-dependent
microvascular disease is seen in a transgenic mouse model of IFN toxicity. This
includes specific microvascular pathological changes seen in patient biopsies
and is dependent on transcriptional activation of the IFN response through the
type I interferon α/β receptor. Together their clinical and experimental
findings provide evidence of a causal link between type I IFN and TMA. So, this
experiment showed that from bedside to bench the clear relationship of
interferon and TMA development.
To
So in
summary, the renal lesions seen with Interferon should really be TMA, and
podocytopathies such as FSGS, collapsing GN and MCD.
Labels:
interferons,
onco nephrology,
pathology,
topic discussions
Friday, November 1, 2019
In the NEWS: NephSim as an educational tool
Nephrology education related published work is sparse. NephSim, a mobile optimized website tool with
cases and interactive approach was developed in 2018. Over 24 cases have been
presented and discussed in this tool. Case contents have been amazing. But what
the creators of this tool now did is- validate it with a peer reviewed
publication. Recently published in JGME, a med ed
journal, Farouk et al showcase the NephSim tool and discuss the results of
their outreach of this tool and a survey that showed high rate of satisfaction
and usability.
Innovation in Nephrology education is extremely important. Case discussions leading to differential diagnosis and then
pathology and diagnosis helps in creating and making a Nephrologist a better
diagnostician. The NephSim project also showcases the use of website, social
media platforms such as twitter and other ways to share information.
This tool can easily be replicated in other fields in
internal medicine or medicine. The ease of using and doing the cases makes it
very accessible and able to be transformed in all fields in medicine. The
drawbacks- survey response was low but enough to make major conclusions. But
like most med-ed studies, it touches the first tier of outcomes- medical
knowledge (self-assessed) and not addressing other ways of medical knowledge.
We hope to see using some of these tools used( perhaps in combo)- such as NephSim, Nephmadness, Whatsapp,
blogs, NephJC. Etc—to change practice patterns,
behaviors and ultimately effect patient outcomes.
Labels:
education,
Fellows,
medical student
Wednesday, October 16, 2019
Saturday, October 12, 2019
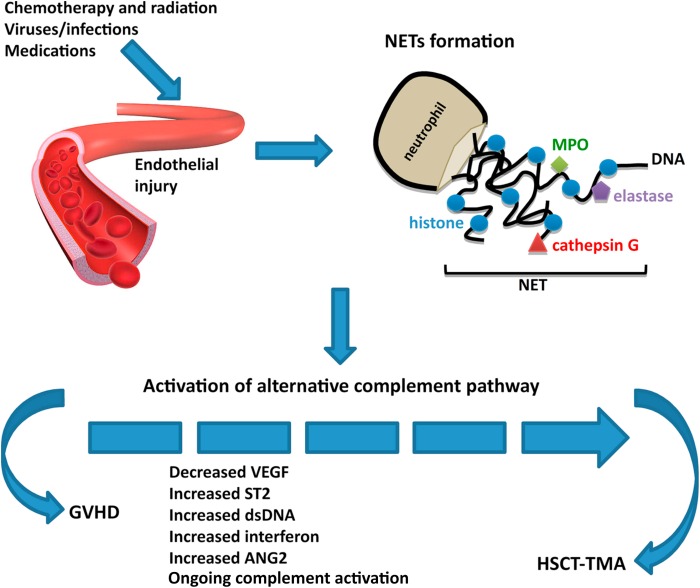
Topic Discussion: HSCT associated TMA, a renal endothelial variant of GVHD
Kidney injury post HSCT is a mystery. While the initial AKI
is from multiple causes, the chronic damage we see in the survivors of HSCT is not well
understood. In a recent
review in AJKD, we did consider this to be mostly TMA related. But is TMA a
form of GVHD ( renal limited) is what some including us have proposed. When one looks at the literature
from GVHD and links to the kidney- one thinks of secondary membranous, but
perhaps this is a rare finding- endothelial glomerular damage might be more
common(TMA).
In a recent mice
study, the authors looked at HSCT effect on kidney in various murine models of
GVHD. The most common finding was glomerular with classic mesangiolysis, mesangial
proliferation and edema with subendothelial widening and microthombi. These are
features of HSCT- associated TMA. So, it is very possible that getting a HSCT
might be a second hit to several folks who might carry a complement deficiency
and perhaps there is some activation of complement system.
Some
of the literature proposes that TMA and GVHD are not related but both affect
the complement cascade. As clinicians we have seen several cases of TMA and
concurrent GVHD and a recent reported case series confirms this. It is
intriguing and possible that renal-limited TMA might be a variant of GVHD. GVHD is usually an epithelial cell disease but
having an “endothelial” target might be possible in the kidney. In most cases,
when TMA is diagnosed in a patient with HSCT, the knee jerk response is to
discontinue CNIs. Whether this is of potential benefit or harm is not clear.
Labels:
basic science,
onco nephrology,
TMA,
topic discussions
Wednesday, October 9, 2019
Tuesday, September 17, 2019
Concept Map: Polyuria
A solute diuresis is defined -- urine osmolality >600 mosmol/kg and a total daily osmolar output >1000 mosmol (calculated as the urine osmolality multiplied by the 24-hour urine output).
A water diuresis is defined with a urine osmolality <600 mosmol/kg and often <300 mosmol/kg and a total daily osmolar output <900 mosmol.
Another way to look at it from pure Uosm perspective is U osm <100mOsm/kg is generally a water diuresis from polydipsia or DI.
Uosm between 100-300 mosm/kg is usually a mixed polyuria( either a central and nephrogenic partial DI and maybe simultaneous water and solute intake and CKD)
U Osm >300 is generally solute diuresis
Labels:
concept maps,
natremia,
polyuria
Thursday, September 12, 2019
Topic Discussion: Do Renal consultations matter in surgical and cardiac ICU patients
AHA moment arrived when I saw this article in AJKD on interdisciplinary
collaboration of nephrology with surgical and cardiac surgery ICUs. It was a
qualitative study highlighting some of the conversations that happen in the
CTICU with the nephrologists and what is “felt” about renal consultations.
This is an important topic that we encounter as consultants.
Often, we get urgent calls from the ICU, for example CTICU , “ Doc, we need an
urgent consult, this patient post CABG is oliguric now and crt rose from 1 to
1.4mg/dl and we need urgent CRRT, and we placed the dialysis catheter already
for you…”
Now this situation is not uncommon… how does one respond to that..
Either you say, “ gee. Thanks for that and I will come evaluate and decide if I even need to use that catheter as they might not need dialysis..” What is the role of the Nephrologist in some of the surgical run ICUs.? Are we seen merely as technicians or truly thoughtful physicians that make decisions that will or not alter the care of the patient..
Now this situation is not uncommon… how does one respond to that..
Either you say, “ gee. Thanks for that and I will come evaluate and decide if I even need to use that catheter as they might not need dialysis..” What is the role of the Nephrologist in some of the surgical run ICUs.? Are we seen merely as technicians or truly thoughtful physicians that make decisions that will or not alter the care of the patient..
The article really highlights this very important issue. Some
of the major themes highlighted are listed below
1.
There was almost an absent influence of renal
decisions in some of the surgical and CTICUs; this stemmed from many surgeons
and intensivists not sure of the renal fellows decisions not going along with
attending nephrologists decisions. In my opinion, many times and at many
centers-they bypass fellow based consult services and call attendings only for
that reason.
2.
Nephrology fellows and attendings found it hard
to communicate to CTICU staff as the PA or NP would not really be making that
decision and the final decision came from the surgical head of that patient (
who often is not in the unit)
3.
Nephrology fellows might not realize the hierarchy
noted in some of the surgically based ICUs compared to MICUs. This is interesting as the first time we
encounter surgical culture in depth is during renal fellowship( 3 years in
medicine- we usually are kept away from SICU, CTICU and NSICU)
4.
What I found totally astonishing was one of the
comments made in box 2 by an NP that was interviewed is that “renal was the
only service we had to call to get something done as We can’t just order
dialysis” – and hence making us seem like just a dialysis ordering physician
5.
It also goes into details on who manages the fluid
removal once CRRT has been started. It is an ongoing battle. Often this leads
to conflict and at many centers, Nephrologists have given up CRRT ordering and management
to ICU intensivists( sad but true)
6.
Due to our consult note and recommendations have
no value- many times- there was early signing off of the consult- as “ if they
are not listening to our recommendations anyway – why bother writing a note everyday…”
Not uncommon to see in this unit.
7.
While Nephrologists thought they were best
valued to understand AKI and noted a good nephrologist is a good internist. Meanwhile,
surgical staff didn’t believe that and felt nephrologists were mostly dialysis
gatekeepers and didn’t feel we understood AKI in the overall ICU status and
ordering tests of diagnostic significance were not very valuable.
8.
The role of nephrologists being dialysis
proceduralist clashed nephrologists value of preventive medicine mainly in the CTICU.
From a surgical perspective, a consultation that doesn’t offer any valuable
intervention such as dialysis to help the acutely ill patient is useless. –
heard that one before many times
9.
The most common disagreements were on when to do
dialysis, timing of initiation and managing fluids—the most common we see in
practice anyway. It is not uncommon where I have written “ stop diuretics” but
they are continued and then days later I am starting them on RRT. But there have been also times where I have
said “ stop diuretics” and they continued and they did better by not listening
to me. So in general, does our opinion matter?
10.
Interesting, surgical and CT ICU staff viewed
dialysis as a tool to get rid of the kidney problem whereas we see it as a last
resort before trying all medical maneuvers. One comment was really funny, In box 3, one of
the nephrologists interviewed said “ they view most of us as technicians. Just
like anesthesia can just put the person to sleep, just put a tube and no big
deal- anyone can do it, you can slap someone on dialysis, no big deal.”. My favorite one I get called is “ can you come
and spin him”
11.
Finally, due to history of these interactions,
nephrologists and nephrology fellows avoided the controversial issues. Many times,
this led to resignations from the case.
12.
Lot of these changes are due to different
medicine vs surgical cultures.
How do we fix this? Can we fix this? The authors describe this
is discipline siloing leading to ineffective collaboration amongst fields of medicine.
This is important to break and learn. This will be critical as it can harm patients
if gets escalated and neglect ensues. We need to understand the other persons perspective
and realize that all physicians have one medical school, residency and fellowship—we
all bring in some value to the patient. We need to respect and honor each other’s
fields of medicine.
When I showed this article to one of our CT surgeons, his/her
reaction was merely to dismiss it. My fellow and I were hoping for more of a
conversation to improve this encounter.
Then the next day, in the CTICU, we see that the curtains
are closed and one of the rooms was having open heart surgery happening in the middle
of the ICU – for an urgent mater. We
were just amazed at the life saving nature of their field in medicine… it is
just amazing what they can do. And I told my fellow, “ if they can make the ICU
bed an OR instantly, their assumption is that dialysis can happen instantly and
at any place- even in the OR..” We have to understand that they come from a different
perspective. Once we start understanding
that, we may be more welcoming of their way of thinking. Similarly, at some
point, perhaps they can understand our physiological approach to certain things
and preventive nature of AKI and that dialysis is a procedure and not the first
thing we should be doing..”
Labels:
cardiac surgery,
education,
General Nephrology,
icu nephrology
Sunday, August 25, 2019
Topic Discussion: Artificial Intelligence in Nephrology
Artificial intelligence(AI) is on a rise in science. Using
it in medicine and specifically nephrology is sure to come.
According to the dictionary, AI is “the theory and development of computer
systems able to perform tasks that normally require human intelligence, such as
visual perception, speech recognition, decision-making, and translation between
languages.”
Dr Eric Topol has been
a big proponent of this concept in medicine for years and recently has written
a book called “Deep
Medicine “ that details the potential uses of this in medicine.
Basically, AI can help
in three main ways: 1) diagnosis that is often challenging in various challenging
syndromes and even basic common ones. 2) make the physician’s life easier and
decrease paper work and finally leading to the third -the most important 3) spending
more time at the bedside.
AI is done via creating
an artificial
neural network (ANN ) which is simply a collection of artificial neurons
organized in layers. In a recent article in
AJKD, authors discuss the potential use of this concept in Nephrology. They
describe using it for IgA nephropathy(IgAN) as a recognizable cause for AKI. The ability to identify the patients that
will progress to ESRD with IgAN would be useful for prognostic and therapeutic
reasons. Geddes
et al hypothesized that there exists a function that associates
clinical and biological parameters measured at the time of IgAN diagnosis (namely
age, sex, blood pressure, proteinuria, serum creatinine level, and
antihypertensive treatments) to the probability of developing progressive IgAN.
The authors designed and implemented an ANN to approximate this function. The
results showed that their ANN could predict the occurrence of progressive IgAN
more accurately than experienced nephrologists (correct predictions, 87% vs
69.4%; sensitivity, 86.4% vs 72%; and specificity, 87.5% vs 66%). Hmm, now this
might be interesting to help guide a lot of therapies in Nephrology. This might
be very useful in transplantation and prognosticating even need for dialysis
for the elderly CKD patients.
Interestingly, many AI
algorithms have been approved by FDA that are used in clinical practice:- some
examples are of Atrial fibrillation detection, EF ECHO determination, Coronary
calcium scoring, CT brain bleed diagnosis, device for paramedic stroke diagnosis,
breast density via mammography to name a few.
No nephrology related such algorithms are approved to my knowledge.
There is an entire
journal dedicated for this in medicine now
Nephrologists, let’s get started and catch on!
Monday, August 5, 2019
Topic Discussion: Osmotic Nephrosis
Osmotic nephrosis describes a morphological pattern with
vacuolization and swelling of the renal proximal tubular cells.
What does the pathology show:
Usually there is acute tubular necrosis–like changes. Histologically,
osmotic nephrosis is characterized by a focal or, less often, diffuse
“clear-cell” transformation of proximal tubular epithelial cells showing
isometric fine vacuolization of the cytoplasm . The straight part of the
proximal tubule primarily is involved and, in severe cases, also the convoluted
part. Severely affected tubules are often seen side by side with
normal-appearing tubules. Distal tubules and collecting ducts are more or less
unchanged
Classic known causes of this entity are:
Intravenous immune globulin preparation(sucrose based)
Mannitol
DextransContrast media
Hydroxyethyl starch
Glucose
How does one differentiate this from vacuolization seen with
tacrolimus and cyclosporine? Is that a form of osmotic nephrosis?
Renal Pathologist Dr Lynn Cornell nicely describes this on
twitter with these images. The image below shows isometric vacuolization in CNI
toxicity. This leads to have focal tubules with this change( see arrow)
In osmotic nephrosis, tends to show vacuolated cytoplasm in
tubules diffusely( see below)
Osmotic nephrosis describes a morphological pattern with
vacuolization and swelling of the renal proximal tubular cells.
In addition, In paraffin sections, the isometric
vacuolization seen in patients with calcineurin-inhibitor toxicity may be
indistinguishable from osmotic nephrosis. However, electron microscopy shows dilated
endoplasmatic reticulum as the cause of vacuolization in the former. Osmotic nephrosis cannot be differentiated
from lipid storage in tubular cells (foam cells), as seen in patients with
nephrotic syndrome, liver failure, or intoxication. In such cases, foam cells
also are often found in large amounts in the interstitial space. This does not
occur in osmotic nephrosis.
The above image shows osmotic nephrosis in a kidney biopsy specimen.
(A, B) Tubular cross-section with seemingly no lumen. Epithelial cells are
massively swollen, cytoplasm is completely filled by vacuoles of about the same
size (isometric vacuoles), and nuclei are displaced to the base of the cells
and distorted by adjacent vacuoles( source https://www.ajkd.org/article/S0272-6386(07)01592-2/pdf
Labels:
osmotic nephrosis,
pathology,
topic discussions
Tuesday, July 23, 2019
In the NEWS: The New Kidney Health Order
Few weeks ago, there was an executive order signed to advance kidney health in the US. This is an historic event for the field of Nephrology and for kidney patients. The above image is a visual abstract that summarizes the changes that might be coming in 2020. This image is courtesy of Dr Tejas Desai @nephondemand
The goal of this order is to increase home dialysis options, increase organ transplantation and promote kidney health and keep patients "away from dialysis". In addition, several incentives have been built in to allow for improved compensation for physicians and what looks like better options for patients. What does this mean for Nephrology?- Time will tell but this is a huge improvement in terms of patient care and patient choices. Hope this also sparks some more interest in the field of nephrology where we are still struggling for trainees.
Saturday, July 13, 2019
Friday, June 28, 2019
Topic Discussion: Amyloidosis and Renal Infarction
Usually when we
think of amyloidosis in the kidney- we think of paraprotein mediated
amyloidosis (AL or AH) leading to nephrotic syndrome and in some rare cases-
vascular amyloid presenting as AKI.
A
recent study published in Mayo Clinic Proceedings suggests that renal
infarction might be a common finding in patients with cardiac amyloidosis. Three groups of patients were
identified according to the underlying amyloidosis disorder: AL amyloidosis in
24 patients, mutated-transthyretin amyloidosis in 24 patients, and wild-type
transthyretin amyloidosis in 39 patients. Patients with AL amyloidosis had
significantly higher N-terminal pro-B-type natriuretic peptide levels (P=.02) and were more
likely to have nephrotic syndrome (P<.001). Renal
infarction was detected in 18 patients (20.7%), at similar frequencies in the
various groups. The likelihood of RI diagnosis was 47.1% (8 of 17) in the
presence of AKI and 14.5% (10 of 69) in its absence (P=.003). Renal infarction
(defined by defect(s) on the DSMA scan) was reported in 20.7% of patients with
and 25% without evidence of cardiac amyloidosis. Prior studies
have not really shown any association like this before of amyloidosis and
infarction. Renal
infarcts were described in an autopsy study in 3 kidneys that had either
cast nephropathy, plasma cell nodules, or autolysis but not with amyloid
deposits. Dang et al interesting are reporting is a high
percentage of abnormal DSMA scans in patients with wild-type
transthyretin amyloidosis (wtATTR) and mutant
transthyretin amyloidosis (mATTR) amyloidosis.
These findings are
intriguing. The 20% to 25% prevalence reported by Dang and colleagues was
therefore unexpected. Renal
involvement in ATTR is thought to be rare, especially in patients with
wtATTR amyloidosis. Recent drugs used to treat this form of amyloidosis might lead to a
glomerulonephritis( my
recent post). The finding from the current study suggests that we may be
vastly underestimating the prevalence of kidney involvement in ATTR amyloidosis. These patients usually don’t
present with nephrotic range proteinuria but more with AKI and subacute AKI.
Perhaps, instead of labeling all of these as cardio-renal syndrome, we should
consider looking for renal infarction in these patients. And as I have always thought about ruling out amyloidosis in young
males who present with renal infarction, I usually stop at AL-AH amyloidosis
testing. Given the above findings, perhaps an amyloid scan to look for wtATTR
and mATTR might be important as perhaps renal infarction could be a potential
relationship here.
Quite an interesting association!!
Thursday, June 20, 2019
Topic Discussion: Tumor Lysis Syndrome with immunotherapy
At this point the nephrology and oncology community is very
aware of the AIN, glomerular diseases including vasculitis and ATN seen with check point inhibitors…
but we might not be aware of tumor lysis syndrome that this drug can entice in
certain patients.
In the last 2 years, I found several published reports of
TLS with check point inhibitors.
Here is a table I created to help with the theme on this one
|
Immunotherapy
|
Age
|
Gender
|
Cancer type
|
Time to TLS
|
Dialysis needed?
|
Outcome
|
Reference
|
|
Nivolumab
|
76
|
Male
|
Melanoma
|
5
|
Yes
but declined
|
Disease
progression-death
|
|
|
Atezolizumab
|
77
|
Female
|
GU
cancer
|
14
|
Yes
|
Disease
progression-death
|
|
|
Atezolizumab
|
-
|
-
|
Solid
tumor
|
-
|
-
|
-
|
|
|
Atezolizumab
|
-
|
-
|
Solid
tumor
|
-
|
-
|
-
|
|
|
Ipilumumab
|
73
|
Male
|
Melanoma
|
6
|
No
|
Death
|
|
|
Nivolumab
|
74
|
Male
|
RCC
|
2
|
No
|
Death
|
|
Based on this, it is seen with PD-1, PDL1 and CTLA4
inhibitors and melanoma and urological cancers. A recent review in JON
showed a case of a patient getting TLS with melanoma. So it’s hard to tell if these agents are
causing it or is it the burden of tumor- usually solid tumors in these cases.
Subscribe to:
Posts (Atom)











