Using Concept Maps and trying to understand what the medical student already knew and then after reading and doing a renal elective, improved on her "concepts".
Friday, September 30, 2011
Thursday, September 29, 2011
The American Society of Transplantation Blog

Welcome to the blog sphere!
American Society of Transplantation starts a blog for interaction with their members!
http://members.a-s-t.org/blog/beginning-conversation
Labels:
AST,
blogs,
E-Nephrology,
education,
kidney transplantation,
transplantation
TOPIC DISCUSSION: SIROLIMUS , not all that benign
The complications that can occur with use of sirolimus are not that benign. The agent is good in certain cases but not all. Besides the known side effects of FSGS, proteinuria and TMA in the kidney, there are other non renal side effects that one needs to be aware of.
1. Leukopenia, Anemia, Thrombocytopenia
2. Impaired wound healing
3. Mouth Ulcers
4. Oligospermia
5. Interstitial Pneumonitis
6. Hyperlipidemia( all increased LDL, HDL and VLDL)
7. Hyperglycemia
Remember to monitor especially for lipids and glycemic control
1. Leukopenia, Anemia, Thrombocytopenia
2. Impaired wound healing
3. Mouth Ulcers
4. Oligospermia
5. Interstitial Pneumonitis
6. Hyperlipidemia( all increased LDL, HDL and VLDL)
7. Hyperglycemia
Remember to monitor especially for lipids and glycemic control
Wednesday, September 28, 2011
In the NEWS: A novel treatment for Pre eclampsia- A renal method
Soluble fms-like tyrosine kinase 1 (sFlt-1), an alternatively spliced variant of the vascular endothelial growth factor receptor 1, induces a preeclampsia-like phenotype in experimental models and circulates at elevated levels in human preeclampsia. This is the factor that leads to decreased VEGF in pre eclampsia and causes the kidney related findings. Mice studies have shown that giving VEGF can alleviate the findings; but giving VEGF might pose significant risk to the fetus. Investigators at Harvard have shown for the first time that sFLT-1 can be removed via apheresis. Dextran sulfate apheresis lowered the levels of sFlt1 and reduced proteinuria, blood pressure and allowed for fetal growth in their observational paper. This pilot study is published in the August Circulation Issue and further testing is needed in a randomized fashion with more subjects to prove this will work.
Labels:
General Nephrology,
In The News,
pre eclampsia,
pregnancy,
sfLT-1,
VEGF
Tuesday, September 27, 2011
Consult rounds: Secondary causes RULE
 When one thinks about glomerular diseases in Nephrology; its interesting that we always have found some secondary causes for everything. All diseases from Membranous GN, Minimal Change, MPGN and igA Nephropathy have known secondary causes and one has to rule them out first. If no cause is found, we hence call it "idiot"iopathic - since we are idiots about the cause.
When one thinks about glomerular diseases in Nephrology; its interesting that we always have found some secondary causes for everything. All diseases from Membranous GN, Minimal Change, MPGN and igA Nephropathy have known secondary causes and one has to rule them out first. If no cause is found, we hence call it "idiot"iopathic - since we are idiots about the cause. If one looks at each disease specifically, IgA Nephropathy probably has the least known secondary causes and MPGN likely has the most known secondary causes. The rest would fall somewhere in between. Perhaps one day- the list of primary idiopathic will be smaller and known causes can be identified.
Of all the glomerular diseases, when one encounter's MPGN, think secondary and go back to the history and see if there are clues to an underlying secondary cause.
IN THE NEWS:- Renal Cell Cancer Research Update 2011
Renal Cell Cancer(RCC) has been in the limelight recently. Two recent studies provided some interesting links. In a large prospective study, regular use of NSAIDs was associated with a 51% increased risk of RCC after adjusting for multiple variables, according to a report in Archives of Internal Medicine 2011 issue.
The absolute risk differences for regular users compared with nonusers of nonaspirin NSAIDs were 9.15 per 100,000 person-years for the women and 10.92 per 100,000 person-years for the men. This was an analysis of over >75,000 patients. In addition, longer use of nonaspirin NSAIDs was associated with increasing risk. This is the largest prospective trial to look at this link.
In another study recently, researchers have identified an association between RCC and multiple myeloma. They looked at over 57,000 patients diagnosed with RCC as a primary malignancy and over 33,000 diagnosed with multiple myeloma as a primary malignancy. The researchers found 88 multiple myeloma cases in the RCC cohort. Multiple myeloma was 1.51 times more likely to be found in RCC patients than in the general population, according to the investigators. They identified 69 RCC cases in the multiple myeloma cohort. RCC was 1.89 times more likely to be present in patients with multiple myeloma than in the general population.
The absolute risk differences for regular users compared with nonusers of nonaspirin NSAIDs were 9.15 per 100,000 person-years for the women and 10.92 per 100,000 person-years for the men. This was an analysis of over >75,000 patients. In addition, longer use of nonaspirin NSAIDs was associated with increasing risk. This is the largest prospective trial to look at this link.
In another study recently, researchers have identified an association between RCC and multiple myeloma. They looked at over 57,000 patients diagnosed with RCC as a primary malignancy and over 33,000 diagnosed with multiple myeloma as a primary malignancy. The researchers found 88 multiple myeloma cases in the RCC cohort. Multiple myeloma was 1.51 times more likely to be found in RCC patients than in the general population, according to the investigators. They identified 69 RCC cases in the multiple myeloma cohort. RCC was 1.89 times more likely to be present in patients with multiple myeloma than in the general population.
The first study is suggests a link we knew all along but a prospective study confirms it. The second study is novel and not a common association usually thought about. In other words, should we be screening for RCC in MM patients or vice versa?
Ref:
Labels:
cancer,
In The News,
myeloma,
NSAIDS,
onco nephrology,
paraproteins,
renal cell cancer,
urology
Monday, September 26, 2011
IN the NEWS: HB -EGF and RPGN
Any research in RPGN and glomerular diseases that can possibly
lead to any new drug development are good to review. A recent study in Nature
Medicine diseases the role of the heparin binding epidermal growth factor like
growth factor in the role of glomerular disease. The researchers in this paper
showed denovo induction of heparin-binding epidermal growth factor–like
growth factor (HB-EGF) in intrinsic glomerular epithelial cells (podocytes) from
both mice and humans with RPGN. HB-EGF induction increases phosphorylation of
the epidermal growth factor receptor (EGFR) in mice with RPGN. In mice without
the activation of HB-EGF and no EGFR receptor activation, course of RPGN is
improved. Conditional deletion of the Egfr gene from
podocytes of mice alleviated the severity of RPGN. Pharmacologic blockaded of
the EGFR also can alleviate RPGN.
When these receptors are taken out of the equation- the disease
doesn't take full form.
There are EGFR receptor antagonist that are being used. Not for RPGN
but for cancers.
The epidermal growth factor receptor is a
member of the ErbB family of receptors, a subfamily of four closely
related the tyrosine kinases. Upregulation has led to colon, breast and
neurological cancers.
There are many anticancer therapeutics directed
against EGFR, including gefitinib and erlotinib for lung
cancer, and cetuximab for colon cancer. Cetuximab is a monoclonal antibody inhibitor. This allows for tyrosine kinase inhibition as well leading to treating the cancer.
As we think these above agents might be
beneficial for RPGN, we have to be careful as the first method is a tyrosine
kinase inhibitor approach which can have renal side effects as pre eclampsia
like syndromes, hypertension and thrombotic microangiopathies.
This is where Oncology and Nephrology are at the
forefront of sharing information. Congrats on a nicely designed study.
Ref:
http://www.nature.com/nm/journal/vaop/ncurrent/full/nm.2491.html
Image source: http://www.mc.vanderbilt.edu/lens/article/?id=204
Labels:
anca vasculitis,
basic science,
EGFR,
glomerular diseases,
HB EGF,
In The News,
onco nephrology,
RPGN
Saturday, September 24, 2011
Hypercalcemia in Pregnancy
Check out a nice review on the NEJM Blog regarding
Hypercalcemia in Pregnancy
Hypercalcemia in Pregnancy
Labels:
calcium,
electrolytes,
NEJM,
pregnancy
Friday, September 23, 2011
Topic Discussion: Diabetic Nephropathy Post Kidney Transplantation
Post Transplant Porteinuria: Differential Diagnosis:
1. Recurrent Glomerular Disease
2. Denovo Glomerular Disease
3. Antibody Mediated Rejection
4. Infection from CMV or Adenovirus
5. Collapsing Glomerulopathy
6. Thrombotic Microangiopathy
7. Diabetic Nephropathy
What is the data and incidence on Diabetic Nephropathy Post transplant?
1. Recurrent Glomerular Disease
2. Denovo Glomerular Disease
3. Antibody Mediated Rejection
4. Infection from CMV or Adenovirus
5. Collapsing Glomerulopathy
6. Thrombotic Microangiopathy
7. Diabetic Nephropathy
What is the data and incidence on Diabetic Nephropathy Post transplant?
80-100% of diabetics who undergo transplant will have histological changes of diabetic nephropathy, which may be seen within 6 years. Diabetic nephropathy is not believed to be a significant cause of allograft failure, but has not been well-studied.
•One study that looked at a 5 year prospective trial of 48 pts with type-1 DM underwent renal transplant
•Patients were randomized to intensive or standard insulin therapy (insulin several times per day/continuous vs 1-2 times daily); statistically significant difference in A1C maintained
. The five year post-transplant biopsy showed standard group had 2x increased volume of mesangial matrix (p=0.024) and 3x increase in arteriolar hyalinosis, wider basement membrane(p < 0.10).
Understudied disease but by far the MOST common cause of proteinuria years following a kidney transplantation.
Post by Prasanth Krish, MD
Renal Fellow, Hofstra NSLIJ
Renal Fellow, Hofstra NSLIJ
Ref:
Topic Discussion: A Fresh Look at The Banff Classification
The Banff Classification and the Singularity in Transplant Pathology
Many of you know I spend a great deal of time talking and thinking about artificial intelligence, exponential technological change and the Singularity, that point in time when machines are more intelligent that we are and technologic change becomes so rapid that we humans have difficulty keeping up with the progress going on around us. I am teaching a course on the Singularity and the Future of Medicine at the University of Alberta and writing a book on the subject to be completed later this year.
So in my role as the transplant pathologist who runs the Banff meetings along with Lorraine Racusen a logical question is: Where or what is the Singularity equivalent in transplant pathology?
In talking about the Singularity we speak about an event horizon we cannot see beyond in future prediction, and so by definition the Singularity is hard to predict.
Nevertheless I think it is clear what the Singularity equivalent in transplant pathology might involve. On the one hand it could be that point in time where our diagnostic methods to tell us what is going on in the transplanted kidney entirely change, where we are using noninvasive and/or genomic methods entirely to make diagnoses in the transplanted kidney. Half of you are now thinking "no, no that will never happen!".
But you have not heard my "on the other hand" statement. On the other hand the Singularity could be the point at which we are no longer doing transplantation because medical science is so advanced that when we need a new organ we just grow one from stem cells, print one using the 3D printer. And now the other half of you are saying "no, no that will never happen".
But when you think about it more deeply you will realize that one or the other of these things will certainly happen and maybe both. Just like with the general Singularity, the only question is when. If indeed the general singularity is 2045 I predict that the Singularity in transplant pathology will be much earlier than that.
Have you noticed that the organs made by tissue engineering so far are a bit misshapen? There will be some natural pathologies in organs grown from stem cells, and the transplant pathologists of today will be central to figuring that out.
So that is my other prediction, that the Banff meetings and the Banff consensus process will be where these dramatic technologic changes are first manifest, and where the means of coping with them will be worked out. So the people who attend the Banff meetings and participate remotely and the new people who join us in future meetings will be central to the management and adaption to the rapid technologic changes we will see in the future.
It that sense what we do in the Banff meetings has broad general applicability to problems of the future. The specific advances and changes we discuss and incorporate in the meeting will also help us in a general way to be prepared for even greater changes in the future.
Some of you may feel that we allow too much shifting sand, we should demand more stability, a slowing of the pace of change. But as Kevin Kelly says in his recent book What Technology Wants technology change has its own relentless momentum. We cannot slow it down, and to prepare ourselves for what is coming in the future we must be prepared at some level to question everything. Remember that old pre WW II adage "Keep Calm and Carry On"? It is now enjoying a rebirth and is once again popular with young people. Well we must do that, maintain stability, while questioning everything, the old and the new. It is a difficult path to follow but also an exciting one. There is a tension there but it is a healthy tension. In the end that combination of stability and constant inquiry is the only logical course forward.
So here is to the future of the Banff process in transplant pathology. onward and upward!
Post by Kim Solez, MD
Transplant Pathologist
Univ of Alberta, Canada
Wednesday, September 21, 2011
TOPIC DISCUSSION: Cirrhosis, Hyponatremia and the role of Vaptans?
CHF and SIADH has clear indications for use of vaptans. What is the data and evidence in cirrhosis associated hyponatremia?
1. No drug has been approved to treat hyponatremia of cirrhotics as of yet
2. Studies from Europe using lixivaptan and satavaptan have shown that short term effects of using them have had increased serum NA levels in cirrhotics and sustained for 1-2 weeks.
3. Unfortunately , only 27-54% had complete normalization of the Na
4. All studies showed increased urinary Na levels
5. Only one study has shown long term effects of captains in cirrhotics and that is using satavaptan.
The main finding was that the improvement in serum Na obtained in first days of therapy was maintained for one year.
6. Thirst was the biggest side effect.
7. To avoid rapid correction, use of D5W after reaching a certain threshold and matching urine output cc by cc is recommended as risk of ODS is there but not been reported thus far.
8. Patients awaiting liver transplantation should get treatment for Hyponatremia as there have been situations were Na corrects rapidly as the transplant is replaced and subsequently causing neurological sequelae.
Ref:
http://onlinelibrary.wiley.com/doi/10.1002/hep.22418/pdf
http://www.ncbi.nlm.nih.gov/pubmed/19797900
http://www.ncbi.nlm.nih.gov/pubmed/12671890
1. No drug has been approved to treat hyponatremia of cirrhotics as of yet
2. Studies from Europe using lixivaptan and satavaptan have shown that short term effects of using them have had increased serum NA levels in cirrhotics and sustained for 1-2 weeks.
3. Unfortunately , only 27-54% had complete normalization of the Na
4. All studies showed increased urinary Na levels
5. Only one study has shown long term effects of captains in cirrhotics and that is using satavaptan.
The main finding was that the improvement in serum Na obtained in first days of therapy was maintained for one year.
6. Thirst was the biggest side effect.
7. To avoid rapid correction, use of D5W after reaching a certain threshold and matching urine output cc by cc is recommended as risk of ODS is there but not been reported thus far.
8. Patients awaiting liver transplantation should get treatment for Hyponatremia as there have been situations were Na corrects rapidly as the transplant is replaced and subsequently causing neurological sequelae.
Ref:
http://onlinelibrary.wiley.com/doi/10.1002/hep.22418/pdf
http://www.ncbi.nlm.nih.gov/pubmed/19797900
http://www.ncbi.nlm.nih.gov/pubmed/12671890
Tuesday, September 20, 2011
CLINICAL CASE 43: ANSWERS and SUMMARY
A 34 Y OLD FEMALE WITH KNOWN IGA NEPHROPATHY ASKS YOU ABOUT GREEN TEA AS A POSSIBLE TREATMENT. WHAT COMPONENT OF GREEN TEA HAS SHOWN THERAPEUTIC POTENTIAL IN GLOMERULAR DISEASES IN MURINE MODELS?
Caffeine
1 (2%)
Theanine
9 (18%)
EGCG( catechin)
25 (50%)
Vitamin B 2
4 (8%)
Saponin
4 (8%)
Chlorophyll
7 (14%)
Green tea has many different components as listed above. The catechin components have shown in mice studies to reduce oxidative stress. It is believed that it's these components of green tea that might benefit in many diseases.
A recent study in Kidney International showed that EGCG( Epigallocatechin-gallate )reversed the progression of immune mediated GN in mice by targeting redox and inflammatory pathways. Another mice study also showed that cisplatin induced rats didn't have the renal damage noted usually with cisplatin when pretreated with EGCG.
The study showed for the first time that EGCG can protect against Cisplatin-induced nephrotoxicity. At the molecular level, cisplatin triggers a high level of oxidative stress and systemic inflammation, events that were all abrogated with EGCG.
The other substances in green tea are also protective but no direct renal protection has been shown. Vitamins are always good. Theanine is an amino acid that promotes neural function balance and perhaps has activity against hypertension. Saponins might have some anti cancer effect and augment the immunity but not well studied. Chlorophyll gives the green pigment to the tea.
Monday, September 19, 2011
Sunday, September 18, 2011
Topic Discussion: Cathepsins and the Kidney
 Investigators are getting more and more interested in Cathepsins
and their role in health and disease. Atherosclerosis and diabetes are
closely associated and both involve extensive degradation of the aortic elastin.
Cathepsins are lysosomal cysteine proteases mainly of three types S, L and B.
There are some data on cathepsins involvement in inflammation and
mortality. Recently, in the Sept 2011 issue of JAMA, higher cathepsin S levels
were associated with increased mortality risk in elderly. Studies have
confirmed that elevated levels of cathepsin S has been associated with
worsening diabetes, obesity and atherosclerosis in mice and human models. And
guess what:- the activity of cathepsin S is regulated at the cellular level by
and endogenous inhibitor none other than our friend- cystatin C.
Investigators are getting more and more interested in Cathepsins
and their role in health and disease. Atherosclerosis and diabetes are
closely associated and both involve extensive degradation of the aortic elastin.
Cathepsins are lysosomal cysteine proteases mainly of three types S, L and B.
There are some data on cathepsins involvement in inflammation and
mortality. Recently, in the Sept 2011 issue of JAMA, higher cathepsin S levels
were associated with increased mortality risk in elderly. Studies have
confirmed that elevated levels of cathepsin S has been associated with
worsening diabetes, obesity and atherosclerosis in mice and human models. And
guess what:- the activity of cathepsin S is regulated at the cellular level by
and endogenous inhibitor none other than our friend- cystatin C.
What about the role of cathepsins in renal diseases. The table
below from a recent JCI journal describes the different types of cathepsins and
what their roles are in many diseases.
There is some data that cathepsin L might be involved in
proteinuric renal disease.
Podocytes are unique cells with a complex cellular organization,
consisting of a cell body, major processes, and foot processes . Cell biologic
and mouse genetic studies revealed that many proteins regulate the plasticity
of the podocyte actin cytoskeleton. The onset of proteinuria, induced by either
LPS or puromycin aminonucleoside is associated with the induction of cathepsin
L expression and activity in podocytes. There is an emerging concept that the
onset of proteinuria represents a migratory event in podocyte foot processes
that is associated by the activation of cathepsin L. Podocyte cathepsin L
expression is increased in a variety of human proteinuric kidney diseases,
ranging from minimal change disease (MCD) to diabetic nephropathy. Cathepsin
L–mediated proteolysis plays a critical role in the development of various
forms of proteinuria. Cathepsin L–mediated degradation of dynamin leads to proteinuria in
mice. The notion that dynamin is required for proper
podocyte structure and function is further supported by the observation that
overexpression of dominant-negative dynamin leads to a loss of podocyte stress
fibers in vitro and development of proteinuria in mice.
(image source:- http://www.jci.org/articles/view/42918)
So basically, cathepsins are becoming more and more important players in renal and other non renal diseases. What is interesting is that cystatin C, which is being billed as a renal function marker is an endogenous inhibitor of cathepsin S and an important regulator. So if someone is in renal failure, there is increased cystatin C, there will be increased inhibition of cathepsin S. Either that will increase the levels ( substrate) or decrease it base on how it binds. Hmm.. is it really cystatin C or is it cathepsin S or other cathepsins. Something to ponder and watch out for more work on this field.
Ref:
http://www.jci.org/articles/view/42918 ( currently freely available)
Friday, September 16, 2011
Consult Rounds: Membranous Post Kidney Transplantation
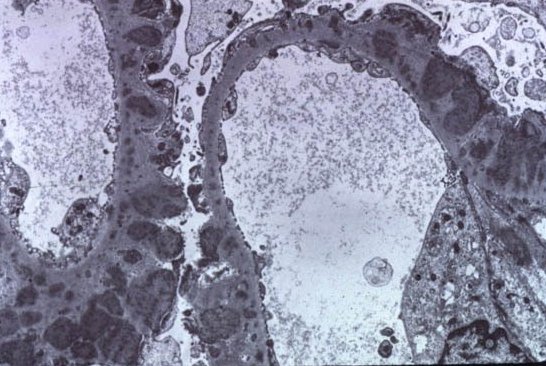 Recurrence of Primary Glomerular Disease is the 3rd most common cause of graft failure.
Recurrence of Primary Glomerular Disease is the 3rd most common cause of graft failure.Membranous GN has a Recurrence post transplant rate of : 30-40% , Usually diagnosed between 2nd-3rd year post transplant. The graft survival is not different than that of patients with other renal diseases. One major study in NDT in 2010 is the only one that really looked at post transplant membranous GN and what happens to those patients. They had 12 patients with recurrence of Membranous. In th 118 month follow up, patient survival was 96% and graft survival was around 40% when compared to controls. Recurrence led to graft loss in 6 patients, all were DDRTx and in 55 months. Recurrence was more common in females. The ones with higher proteinuria did worse. This showed that they did similar patterns as regular Membranous GN.
Another study done recently in 2010 showed that recurrence might be lower compared to previously thought due to being on strong anti rejection agents. The incidence of recurrent iMN was 44%, and recurrences occurred at a median time of 13.6 months after transplantation. Two patterns of recurrence were identified: Early and late. No predictors of recurrence or disease progression could be identified. Anti CD20 agents might be useful in treating this post recurrence.
So really, no good overall data out there. Here are a list of references.
Post by Hitesh Patni, MD
Dr Patni is a renal fellow at the Hofstra NSLIJ School of Medicine
Ref:
http://www.ncbi.nlm.nih.gov/pubmed/12110738
http://www.ncbi.nlm.nih.gov/pubmed/21030574
http://www.ncbi.nlm.nih.gov/pubmed/20466669
http://www.ncbi.nlm.nih.gov/pubmed/20185599
Thursday, September 15, 2011
IN THE NEWS:- Myeloproliferative neoplasms and glomerular disease
A recent article referenced below in Kidney International Sept
2011 issue reveals a novel glomerular disease associated with
myeloproliferative neoplasms( CML, polycythemia vera, essential thrombocytosis,
mastocytosis, primary myelofibrosis and other) that usually become AML.
A description of 11 cases showed following characteristics:
1. mesangial scloerosis
2. hypecellularity
3. Segmental sclerosis
4. Chronic TMA findings
5. Intracapillary hematopoietic cell infiltration
6. No signs of immune complex mediated process by EM and IF
The term MPN related glomerulopathy is suggested. This is
interesting to note as clinically I have always wondered about the nephrotic
syndrome that is associated with these cases of MPN. In the literature, there
is information about the MPGN pattern of injury seen with such cases and
perhaps this is just semantics but looking at the findings suggests a
thrombotic microangiopathy and MPGN pattern of injury with novel findings
mainly of the cell infiltration( which might have been there in prior cases
published but not looked for ).
Morphologically, have to be differentiate from diabetic glomerular
changes, FSGS, TMA, and immune complex MPGN
Labels:
cancer,
glomerular diseases,
In The News,
MPN,
onco nephrology,
topic discussions
Wednesday, September 14, 2011
Consult Rounds: Hyperphosphatemia
In the steady state, the serum phosphate concentration is primarily determined by the ability of the kidneys to excrete dietary phosphate. When you give an acute phosphate load over several hours, transient hyperphosphatemia can ensue.
There are three general circumstances in which this occurs: massive acute phosphate load; chronic renal failure; and a primary increase in proximal phosphate reabsorption. The fourth is pseudohyperphosphatemia.
1. CAUSES OF Phosphate Load to consider:
Tumor lysis syndrome
Rhabdomyolysis
Lactic acidosis
Ketoacidosis
Phosphate Enemas or laxatives
Vitamin D Toxicity
2. RENAL FAILURE is self explanatory
3. Increased Tubular Reabsorption of Phosphate:
Hypoparathyroidism
FGF-23 Deficiency
Acromegaly
Bisphosphanates
Tumoral Calcinosis
4. Pseudohyperphosphatemia from elevated bilirubin, paraproteins, lipids and hemolysis
Hope this simplifies the differential of hyperphosphatemia
There are three general circumstances in which this occurs: massive acute phosphate load; chronic renal failure; and a primary increase in proximal phosphate reabsorption. The fourth is pseudohyperphosphatemia.
1. CAUSES OF Phosphate Load to consider:
Tumor lysis syndrome
Rhabdomyolysis
Lactic acidosis
Ketoacidosis
Phosphate Enemas or laxatives
Vitamin D Toxicity
2. RENAL FAILURE is self explanatory
3. Increased Tubular Reabsorption of Phosphate:
Hypoparathyroidism
FGF-23 Deficiency
Acromegaly
Bisphosphanates
Tumoral Calcinosis
4. Pseudohyperphosphatemia from elevated bilirubin, paraproteins, lipids and hemolysis
Hope this simplifies the differential of hyperphosphatemia
Labels:
Consult Rounds,
electrolytes,
phosphate
Tuesday, September 13, 2011
In The News:- HIV Nephropathy and other adverse risk factors
Incidence of HIV-associated
has been decreased significantly after advent of HAART. We hypothesized that
patients who has sustained podocyte HIV expression before the start of HAART
are vulnerable to develop HIV-associated nephropathy during the development of
adverse host factors such as the activation of renin-angiotensin-sytem (because
of the development of diabetes or loss of critical nephron mass as a result of
nephrotoxic drugs or trauma etc) , despite having undetectable viral load. Since
HIV patients are now living almost a normal life and are prone to develop
diabetes and hypertension, they are likely to develop the activation of the RAS
in their later life. To test our hypothesis, HIV transgenic Vpr mice (which display
doxycycline [Doxy] specific podocyte Vpr expression) with 2, 3, and 4
angiotensinogen (Agt) copies (Vpr-Agt-2, Vpr-Agt-3, and Vpr-Agt-4) were
administered Doxy for 3 weeks to manifest clinically occult (in situ) HIVAN
followed by Doxy-free water during the next 3 weeks. Subsequently, renal
biomarkers were collected and kidneys were harvested for renal histology. Vpr
mice with Agt copies did not develop proteinuria and blood pressure, and
displayed minimal glomerular and tubular lesions only, without any microcyst
formation. Vpr mice with 3 Agt copies showed mild glomeular and tubular lesions
and microcyst formation; whereas, Vp mice 4 Agt copies exhibited moderate
proteinuria, hypertension, glomerular sclerosis, tubular dilatation, microcysts
and expression of epithelial mesenchymal transition markers. Moreover, Vpr mice
4 Agt copies displayed enhanced renal tissue expression of Agt, renin, and ACE and
also showed higher (P<0.04) renal tissue concentration of Ang II. In
addition, renal cells in Vpr mice 4 Agt copies showed enhanced expression of
TGF-β, connective tissue growth factor, and vascular endothelial growth factor
(VEGF). These findings indicate that adverse host factors such as the
activation of the RAS, promotes the progression of clinically occult HIVAN to overt
HIVAN despite the absence of active viral activity. Thus, to prevent HIVAN
early detection of HIV infection (prior to renal cell infection) and treatment
would be important.
Post by Pravin Singhal, MD
Dr. Singhal is a NIH funded investigator in the Division of Kidney Diseases and Hypertension at the Hofstra NSLIJ School of Medicine, NY
Dr. Singhal is a NIH funded investigator in the Division of Kidney Diseases and Hypertension at the Hofstra NSLIJ School of Medicine, NY
Labels:
basic science,
glomerular diseases,
HIVAN,
In The News,
infections,
VEGF
Monday, September 12, 2011
Topic Discussions: Genome-wide association studies (GWAS) in CKD
CKD has a heritable
component. Atleast partly this is due to the heritability of diabetes and
hypertension; though racial predeliction in African-Americans, asian
susceptibility to IgA are all examples of further genetic links to CKD. In
Nature medicine, a GWAS attempted to identify susceptibility loci to (i) CKD
(eGFR Creat < 60 ml/min), (ii) eGFR creatinine and (iii) eGFR CystatinC. GWA studies are
usually structured in four parts (1) collection of a large number of blood
sample from individuals with the disease/trait of interest and from a control
group; (2) DNA isolation and genotyping; (3) statistical tests for associations
between the SNPs and the disease/trait; (4) replication of identified associations
in an independent population sample and examination of functional implications
experimentally. This study used four populations-based cohorts from prior
studies (ARIC, CHS, FHS, RS; n=19,877;
with 2,388 CKD cases). They then validated the associations identified in 21,466 independent participants (with
1,932 CKD cases).
Seven SNPs at or
upstream of the Uromodulin (Tamm-Horsfall protein) locus emerged most strongly associated with
CKD . These SNPs were also associated
with both eGFR creat and eGFR Cystatin.
The minor T allele at rs12917707 was associated with a 20% reduced risk
of CKD. UMOD mutations are associated with autosomal-dominant forms of kidney
disease, medullary cystic kidney disease type 2 (MCKD2), familial juvenile
hyperuricemic nephropathy (FJHN), and glomerulocystic kidney disease (GCKD).
Oddly, in UMOD knockout mice, decreased creatinine clearance has been observed
but without significant kidney damage. These mice develop increased
calcium-oxalate urolithiasis with high calcium diets and appear to be more
susceptible to UTI. These populations included DM-2 and HTN – both of which
cause significant glomerular changes. It appears however, that SNPs in UMOD, a
tubular protein, are most strongly
associated with eGFR.
With eGFR-creatinine,
a SNP in Shroom3, an actin binding protein emerged next strongly associated
after UMOD. This SNP is intronic. Homozygous Shroom3 deficient mice die
neonatally from open neural tube defects. In general, shroom3 appears to help
modulate epithelial morphogenesis; but the association with kidney disease
needs to be studied further. SNPs in GATM – gene coding for a protein important
in creatine synthesis were significantly related to eGFR Creatinine. An
intronic SNP between Cystatin C and Cystatin 9
emerged as associated with eGFR Cystatin. Again these SNPs are likely to
represent abnormalities in biosynthesis rather than decreased excretion and
CKD.
Ref:
Post by Madhav Menon, MD
Dr. Menon is a Renal Fellow in training at the Mount Sinai School of Medicine, NY
Labels:
CKD and ESRD,
General Nephrology,
genetics,
GWAS,
topic discussions,
UMOD
Saturday, September 10, 2011
TOPIC DISCUSSION: Atypical HUS
Hemolytic uremic syndrome (HUS) is the
most common cause of pediatric acute renal failure, and need for renal
replacement therapy, affecting between 0.2 and 4.28 people per 100,000
worldwide. Term “Typical HUS” commonly used by paediatricians is referred to be with
a preceding prodrome of a diarrhea (D+). Typical HUS representing >90% of
HUS cases, and mainly found in childhood. This form of HUS follows
gastrointestinal infection with enterohemorrhagic Escherichia coli (EHEC), as a
complication of the infection with Shiga toxin–producing bacteria. These patients require adequate care includes
intravascular fluid replacement to improve the perfusion of affected organs,
primarily brain, gut, and kidneys. Supportive treatment of HUS includes the
transfusion of red blood cells (PRBC) and platelets. Antibiotic therapy of EHEC
infection is unnecessary as the intestinal infection is self limiting and
non-invasive, and antibiotics might encourage bacterial release of Stx and
increase the clinical risk of HUS. Shigella related HUS requires prompt therapy
with appropriate antibiotics.
“Atypical HUS,” indicates a presentation
of HUS without preceding diarrhea (D-), is a misnomer. HUS caused by EHEC
colonisation, without an antecedent diarrhea is known, and on the other hand,
complement mediated HUS is known to be triggered by a diarrheal prodrome. It may be caused by pneumococcal pneumoniae
or HIV, complement dysregulation, drugs (quinine, calcineurin inhibitors,
chemotherapy), other pathologies (malignancy, systemic lupus erythematosus, and
antiphospholipid antibody syndrome), or, rarely, to defective cobalamin
metabolism in infants.
During the last 10 years, it has become
evident that atypical HUS is strongly associated with mutations or polymorphism
in proteins implicated for activation or regulation of the alternative pathway
of complement with the Factor H heterozygous mutations representing the major
cause of such HUS. Defects in the plasma and membrane-bound proteins such as
complement factor H (FH) and the FH related proteins (CFHRs), factor I (FI),
membrane cofactor protein (MCP, CD46), and recently thrombomodulin (THBD) play
a major role in the pathogenesis of this atypical HUS. Anti- Factor H
autoantibodies represent a significant etiology of atypical HUS (newly
discovered), mainly in preadolescent children, but may also be present in
adults. This form of HUS is frequently associated with a particular genetic
status consisting frequently of unequal recombination occurring in the CFH
family locus. Recent guidelines for initial therapy from the European Pediatric
Study Group for HUS recommend starting plasmatherapy as early as possible,
within 24 hours of presentation. This is justified by the frequent rapid
deterioration of renal function in patients with CFH, combined CFI, C3, and CFB
mutations (and the possibility of anti-CFH antibodies).
In conclusion, HUS is not a benign
disease. Even the so-called “classical” D+ HUS has substantial long-term
morbidity. The incidence of Atypical HUS is on the rise, with better
understanding of the pathogenesis of the disease. New strategies are emerging
including the exciting response shown to Eculizumab by the patients with EHEC
0104:H4 epidemic in Germany and Europe. More newer therapies are urgently
needed for this devastating disease.
Labels:
aHUS,
General Nephrology,
onco nephrology,
pediatrics,
TMA,
topic discussions
Friday, September 9, 2011
Topic Discussion: Recent Advances in Acute Kidney Injury
Our understanding of the pathophysiology of acute kidney injury (AKI) has advanced substantially and we envision that in the not too distant future we may be able to identify AKI much earlier than we are able to do so today and that we will be able to institute therapies that can either limit renal damage or facilitate recovery, potentially through measures such as the administration of growth factors, mesenchymal stem cells and others. However, our current therapies still consist largely of supportive interventions alone such as attempts to manage fluid and electrolyte balance and dialysis.
Despite many years of caring for patients with AKI it has been difficult to obtain answers to some relatively simple questions and sometimes what may sound like a logical approach may prove not to be the case. One such question has been regarding the use of fluids and diuretics. Administering fluids to help support blood pressure and hopefully cardiac output and renal perfusion might appear to be a sound approach yet there has been concern that the development of fluid overload might counter any such potential benefit by leading to pulmonary edema and other adverse events. Diuretics might seem to be beneficial as well. They can decrease the work of the kidney, hence potentially limiting oxygen consumption, and in some patients they may increase urine output (thereby “converting” the patient from oliguric to nonoliguric AKI). However, to date diuretics do not appear to improve outcomes in patients with AKI and may actually predispose to its development (radiocontrast nephropathy being one such example). Hence despite decades of experience with fluid and diuretic therapy in patients with AKI this has been an area of uncertainty.
Recent work by Grams et al in CJASN (1) has provided some insights. Using data from the Fluid and Catheter Treatment Trial, which was a multicenter, randomized controlled trial evaluating a conservative versus a liberal fluid management strategy in patients with acute lung injury, these authors investigated the impact of fluid balance post-AKI and diuretic use on mortality. The authors found that having a higher post-AKI fluid balance was associated with a higher mortality rate and that the use of furosemide did not impact on mortality after taking into account the patients’ fluid balance post AKI. The authors did not find any dose of furosemide above which there was an increase in mortality.
It is not uncommon that it may take many years to obtain data for what seem to be straightforward clinical questions. Another example of this is the recent New England Journal of Medicine study comparing diuretic strategies in just 308 patients with decompensated heart failure (2). While one might have assumed that there was an abundance of prospective data in the literature on this issue, there was not. These findings highlight the critical importance of well designed clinical research studies to help advance patient care and the need for more trainees and junior faculty to involve themselves in high quality clinical research.
- Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Clin J Am Soc Nephrol 2011; 6:966-73
- Felker GM, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364:797-805.
Post written by :
Joseph Mattana, M.D.
Dr Mattana is the Chief of Nephrology at the Hofstra NSLIJ School of Medicine, NY
Dr Mattana is the Chief of Nephrology at the Hofstra NSLIJ School of Medicine, NY
Wednesday, September 7, 2011
Nephrology Fellows Boot Camp
Back by popular demand from last year, New York Society of Nephrology is doing a first year renal fellows boot camp again.
Check out the topics and timings of the one day power conference.
http://www.nysn-online.org/BOOTCAMP/Bootcamp_Schedule%206_September_9%2020111.pdf
Check out the topics and timings of the one day power conference.
http://www.nysn-online.org/BOOTCAMP/Bootcamp_Schedule%206_September_9%2020111.pdf
Labels:
conference,
education,
fellows boot camp
Tuesday, September 6, 2011
IN the NEWS:- TIM-1 + B cells? and B cell tolerance!
TIM-1 is a costimulatory molecule that regulates immune responses by modulating CD4+ T cell effector differentiation. A recent mice study in JCI showed that it is expressed mainly in B cell subpopulation. And these TIM-1 positive cells that are B cells also produced IL-10. IL-10 is a very important regulatory molecule like Foxp3. These B cells were characterized as CD1dhiCD5+. Some consider these as B cells that have regulatory function. TIM-1+ B cells were highly enriched for IL-4 and IL-10 expression, promoted Th2 responses, and could directly transfer allograft tolerance. Prior studies have shown such cells in mice to help keep SLE quiet and inflammation under control. Perhaps, just like the T regs, there is a small but strong population that are present called B reg cells. Now studies so far have only found these in mice. Can this hold true in human studies is hard to tell yet. Perhaps! It will be important in the field of transplantation to sought this out as we move forward towards a better world of transplant tolerance. Why do some people have more tolerance than other? Perhaps its a Breg phenomenon. The current study suggests that TIM-1 may be a novel therapeutic target for modulating the immune response and provide insight into the signals involved in the generation and induction of Bregs.
Check it out
http://www.ncbi.nlm.nih.gov/pubmed/21821911
Check it out
http://www.ncbi.nlm.nih.gov/pubmed/21821911
Monday, September 5, 2011
Happy Teacher's Day to all
Today Sept 5th is Teacher's Day in India. Teachers and Educators are considered a very important part of our training as physicians. We have role models from high school, undergraduate, medical school and finally in residency and fellowship: teachers make what we are today.
Teachers come in many forms. Some as your course directors, some as your friends, some as your Attendings on the wards. They make a very important part of your decision making of what you choose as a career in medicine. Nephrology as a field needs more Role Models and teachers. Join today and make teachers day memorable by saying a big thank you to all that taught us throughout our career as we move on. A special thanks to our patients:- our best and most important teachers.
In India, Dr. S Radhakrishnan( second president of the country's) birthday is considered Teachers Day. On such a day, senior students take role of the teacher and pass on the torch. Dr. Radhakrishnan was a philosopher and scholar in comparative religion and philosophy. In USA, May 3rd week is regarded as the National Teacher's Week.
Teachers come in many forms. Some as your course directors, some as your friends, some as your Attendings on the wards. They make a very important part of your decision making of what you choose as a career in medicine. Nephrology as a field needs more Role Models and teachers. Join today and make teachers day memorable by saying a big thank you to all that taught us throughout our career as we move on. A special thanks to our patients:- our best and most important teachers.
In India, Dr. S Radhakrishnan( second president of the country's) birthday is considered Teachers Day. On such a day, senior students take role of the teacher and pass on the torch. Dr. Radhakrishnan was a philosopher and scholar in comparative religion and philosophy. In USA, May 3rd week is regarded as the National Teacher's Week.
Labels:
education,
non teaching,
teachers day
TOPIC DISCUSSION: Hemolysis and Renal dysfunction
What does one think of when you have hemolysis and Acute renal injury?
The differential diagnosis is usually:- HUS, atypical HUS, TTP( all variants), hemolytic anemia causes such as paroxysmal nocturnal hemoglobinuria, autoimmune hemolytic anemia and infectious anemia in malaria or babesiosis.
It is important to think of above causes as hemolysis has been identified. A recent Kidney International case presents Babesiosis as a cause of acute renal dysfunction.
Things to look out for: Elevated indirect bilirubin, LDH elevated, downtrending haptoglobin, peripheral smear showing schistocytes and an elevated reticulocyte count.
Check it out:
Ref: http://www.ncbi.nlm.nih.gov/pubmed/21878956
image source: Wikipedia.com
The differential diagnosis is usually:- HUS, atypical HUS, TTP( all variants), hemolytic anemia causes such as paroxysmal nocturnal hemoglobinuria, autoimmune hemolytic anemia and infectious anemia in malaria or babesiosis.
It is important to think of above causes as hemolysis has been identified. A recent Kidney International case presents Babesiosis as a cause of acute renal dysfunction.
Things to look out for: Elevated indirect bilirubin, LDH elevated, downtrending haptoglobin, peripheral smear showing schistocytes and an elevated reticulocyte count.
Check it out:
Ref: http://www.ncbi.nlm.nih.gov/pubmed/21878956
image source: Wikipedia.com
Sunday, September 4, 2011
The Kidney Doctor: IMAGE OF THE MONTH
The Kidney Doctor: IMAGE OF THE MONTH: This is a patient with edema and an albumin creatinine ratio of 5.2. What cast is this? Answer for August's image of the month: B. Cys...
Labels:
images,
kidney doctor,
other blogs,
pathology
Friday, September 2, 2011
CLINICAL CASE 42 : ANSWERS AND SUMMARY
Which of these is an indication for peritoneal catheter removal with DELAYED replacement back again?( as oppose to simultaneous replacement)- select multiple answers
Refractory Peritonitis
|
20 (35%)
|
Fungal Peritonitis
|
40 (70%)
|
Repeat Peritonitis
|
12 (21%)
|
Relapsing Peritonitis
|
15 (26%)
|
Enteric Peritonitis with clear bowel source
|
21 (36%)
|
Refractory exit site infection
|
24 (42%)
|
Enteric peritonitis with clear bowel source, Fungal peritonitis( which most of you got)
Indications for peritoneal catheter removal With simultaneous replacement are
Relapsing peritonitis,Repeat peritonitis and Refractory exit site infection.
Ref:
http://www.ncbi.nlm.nih.gov/pubmed/21801223
Subscribe to:
Posts (Atom)
All Posts
-
▼
2011
(370)
-
▼
September
(28)
- Proteinuria: From a Medical Student's view
- The American Society of Transplantation Blog
- TOPIC DISCUSSION: SIROLIMUS , not all that benign
- In the NEWS: A novel treatment for Pre eclampsia- ...
- Consult rounds: Secondary causes RULE
- IN THE NEWS:- Renal Cell Cancer Research Update 2011
- A casual reminder; Abraham Verghese: A doctor's to...
- IN the NEWS: HB -EGF and RPGN
- Hypercalcemia in Pregnancy
- Topic Discussion: Diabetic Nephropathy Post Kidney...
- Topic Discussion: A Fresh Look at The Banff Classi...
- TOPIC DISCUSSION: Cirrhosis, Hyponatremia and the ...
- CLINICAL CASE 43: ANSWERS and SUMMARY
- Journal Club: SuPAR and FSGS
- Topic Discussion: Cathepsins and the Kidney
- Consult Rounds: Membranous Post Kidney Transplanta...
- IN THE NEWS:- Myeloproliferative neoplasms and glo...
- Consult Rounds: Hyperphosphatemia
- In The News:- HIV Nephropathy and other adverse ri...
- Topic Discussions: Genome-wide association studies...
- TOPIC DISCUSSION: Atypical HUS
- Topic Discussion: Recent Advances in Acute Kidney ...
- Nephrology Fellows Boot Camp
- IN the NEWS:- TIM-1 + B cells? and B cell tolerance!
- Happy Teacher's Day to all
- TOPIC DISCUSSION: Hemolysis and Renal dysfunction
- The Kidney Doctor: IMAGE OF THE MONTH
- CLINICAL CASE 42 : ANSWERS AND SUMMARY
-
▼
September
(28)







