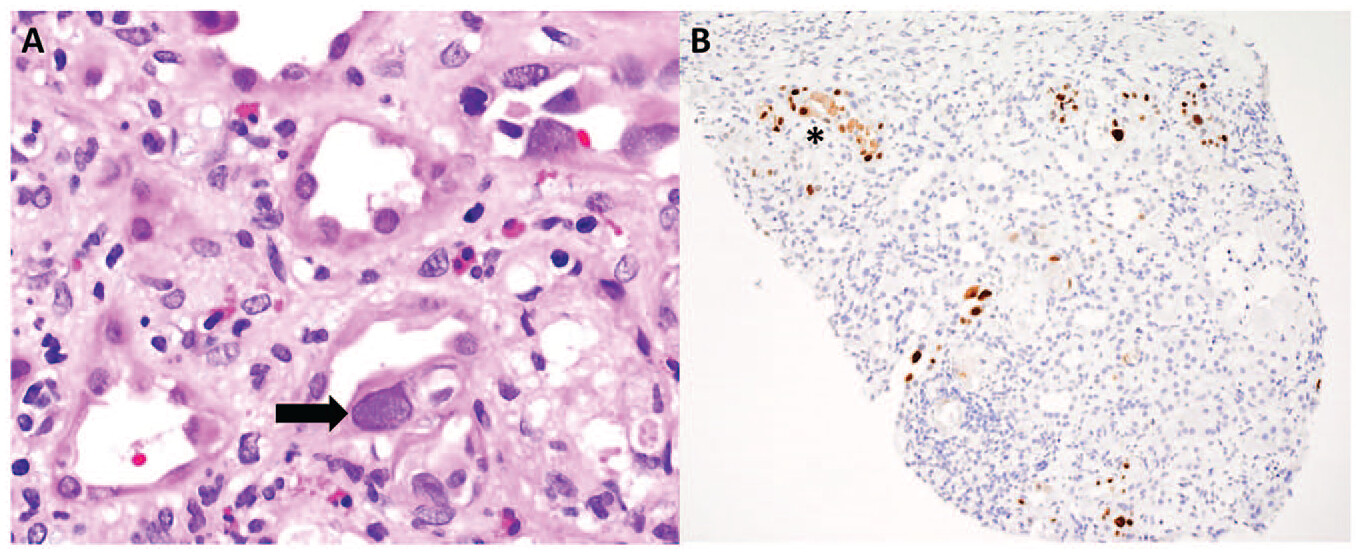
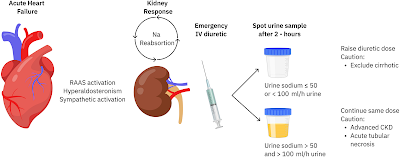
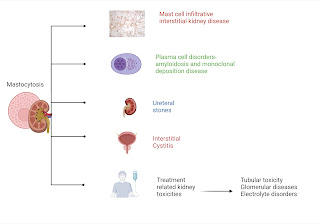
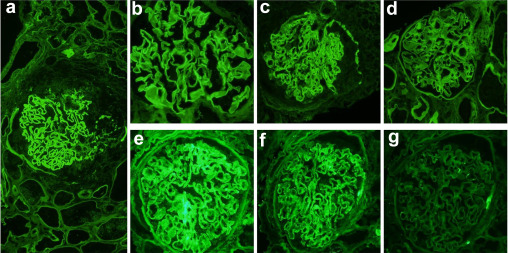
Not much has been written regarding the role of a nephro-hospitalists in the Nephrology literature. There is one perspective back in 2019, before the pandemic that discusses the evolution of nephrology as a medical specialty and addresses the challenges it faces, particularly the declining interest among medical trainees in pursuing careers in nephrology. The authors emphasize the importance of adapting to these challenges and propose a solution in the form of a nephrology hospitalist model.
The field of nephrology has evolved significantly from its early focus on kidney physiology to becoming an independent clinical specialty, particularly with the introduction of dialysis in the 1960s. While patient care was initially delivered primarily in hospitals, the growing population of individuals with kidney disease led to a shift in care to outpatient settings, with a recent emphasis on subspecialized training in transplantation, interventional, and critical care nephrology.
The decline in interest among medical trainees in nephrology careers is attributed to various factors, including a lack of mentorship, the complexity of kidney physiology, busy workloads, perceived lower compensation, and a perceived lack of innovation in therapies and dialysis.
To address these challenges, the authors introduce the concept of a nephro-hospitalist model, exemplified by the experience at Washington University in St. Louis. The model involves a dedicated nephrology hospitalist service comprising attending physicians focusing on inpatient care and medical education. Medical students and rotating internal medicine residents are preferentially placed on this service, and the model includes a flexible schedule of alternating periods of service.
The benefits of this model include improved teaching and mentorship for trainees, increased elective time for fellows, and the opportunity for attending physicians to foster specific interests. The authors highlight the positive impact on education and mentorship, which is crucial for attracting trainees to nephrology.
However, the authors also acknowledge the downsides to the model, including the need for an every-other-month schedule to prevent burnout and potential limitations in attracting new trainees. Financially, the model is described as roughly break-even, and the authors note that financial considerations should be weighed against the educational benefits.
The paper discusses other institutions that have adopted similar models with varying success and mentions the potential role of nephrology hospitalists in private practices, particularly to mitigate issues related to "windshield time" and electronic health record systems.
What have other fields done
Check out this regarding the role of onco-hospitalists and cancer hospitals.
Other fields such as GI and Neurology as well have adopted this model.
It is possible that a full-time hospital-based nephrology model can be a valuable addition to nephrology education, providing increased attending contact and mentorship for residents and medical students. We should consider further exploration of innovative models to expose trainees to the unique aspects and satisfactions of nephrology, ultimately aiming to address workforce challenges and recruit future nephrologists.