Chronic
kidney disease (CKD) is a major global public health problem. In the US, about 11% of adults have CKD as of
2012, and CKD accounts for $41 billion in Medicare expenditures (17%). When patients with CKD progress to end-stage
renal disease (ESRD), the options for treatment are limited to dialysis and
kidney transplantation. Dialysis is
associated with significant morbidity and mortality, and kidney transplantation
is limited by the supply of organs as well as the need for patients to take
immunosuppressive medications for the rest of their lives. There is a need for new, innovative therapies
to treat CKD and ESRD. One promising
approach is to rebuild or repair cells, tissues, or organs to restore proper
function. This exciting new area of
medicine has been termed “Regenerative Medicine.”
We have
been working for the last seven years on developing strategies to differentiate
human pluripotent stem cells, particularly human embryonic stem (ES) cells and
human induced pluripotent stem (iPS) cells, into cells of the kidney lineage
for the purposes of kidney regeneration and kidney disease modeling. We believe that the successful derivation of
functional kidney cells and structures from human pluripotent stem cells will
have an enormous impact on a variety of clinical and translational
applications, including kidney tissue bioengineering to replace lost kidney
tissue, renal assist devices to treat acute and chronic kidney injury, drug
toxicity screening, screening for novel therapeutic agents, and human kidney
disease modeling.
Our primary
goal was to develop a highly efficient, chemically defined method of
differentiating human pluripotent stem cells into kidney tissue. The normal kidney consists of approximately
one million nephrons (the functional units of the kidney). During normal kidney development, nephron
progenitor cells (NPCs) give rise to nearly all the epithelial cells of
nephrons. Nephrons are highly complex
structures with multiple segments, each of which performs a set of specific
physiologic functions of the kidney such as salt and water regulation and waste
product elimination. While previous
studies, including work from our own lab, have demonstrated the ability to
generate NPCs from human pluripotent stem cells, efficiencies have been
low. Furthermore, while these NPCs have
been able to differentiate into rudimentary structures of the nephron, none of
the prior studies have demonstrated the ability to form a complete, mature
nephron from NPCs.
We
hypothesized that a much higher efficiency of NPC generation and formation of
kidney units could be achieved by following nature’s normal differentiation
pathway. We therefore set out to establish a differentiation protocol that
would mimic the stages of nephron formation as closely as possible. Our approach in recapitulating the steps of
kidney development as precisely as possible resulted in a highly robust recipe
for generation of kidney organoids. To our knowledge, this is the most
efficient method for generating complex kidney structures from human
pluripotent stem cells. The ability to do this using induced pluripotent stem
cells, which are derived from skin or blood cells of patients, allows creation
of kidney tissue without ethical concerns and allows the tissue to be
“personalized”, that is, generated from a particular patient. If in the future the tissue is re-implanted
back into the patient, the immune response may then be very limited since the
tissue will be recognized as self.
Finally,
we tested our nephron organoids for the ability to model human kidney
development and drug toxicity to the kidneys.
Kidney development is an important medical topic since it has been
increasingly recognized that individuals can be born with fewer functional
kidney units and these patients are plagued by an increased chance of
hypertension and kidney disease in later life.
By altering the environment of the NPC-derived renal vesicles with drugs
that are known to affect kidney development, we found that the proximal tubule
structures are greatly affected. This finding indicated that the nephron organoids
are usable for the study of human kidney development, for which no “ex vivo” models
currently exist. With this model system
we have a tool to evaluate potential therapeutic agents.
In
addition, we tested nephron organoids for drug toxicity. The kidney organoids were treated with the nephrotoxicants
gentamicin and cisplatin. Both
nephrotoxicants induced segment-specific injury to nephron structures within
organoids in a pattern that is consistent with what is observed in the clinical
setting. Given the individual variation
in drug sensitivity in humans, the generation of these nephron organoids from
human iPSCs would enable drug testing in a patient-specific manner.
Kidneys
are the most commonly transplanted organs, but demand far outweighs
supply. While the human kidney does have
the capacity to repair itself after injury, it is not able to regenerate new
nephrons, the individual functional units that make up the kidney. Human
pluripotent stem cells are the only human cells we can grow in the laboratory
with the potential to generate new functional kidney tissue. Previously,
researchers have been able to differentiate pluripotent stem cells into heart,
liver, pancreas, or nerve cells by adding certain chemicals, but it has been
challenging to turn these stem cells into kidney. Using normal kidney
development as a roadmap, we developed the most efficient method for converting
human pluripotent stem cells into kidney stem cells that will give rise to
nearly all the functional cells of the kidney. These kidney stem cells organize
into mature kidney structures that resemble the structures found in a normal
human kidney. This gives us hope that, one day, we might be able to create
kidney tissues that could function in a human patient and would be 100%
immunocompatible with that patient.
Ryuji Morizane, MD, PhD
Postdoctoral fellow, Renal Division, Brigham and WOmen's Hospital
Albert Q. Lam, MD
Associate Physician, Renal Division, Brigham and Women's Hospital
Joseph V. Bonventre, MD, PhD
Chief, Renal Division, Brigham and Women's Hospital


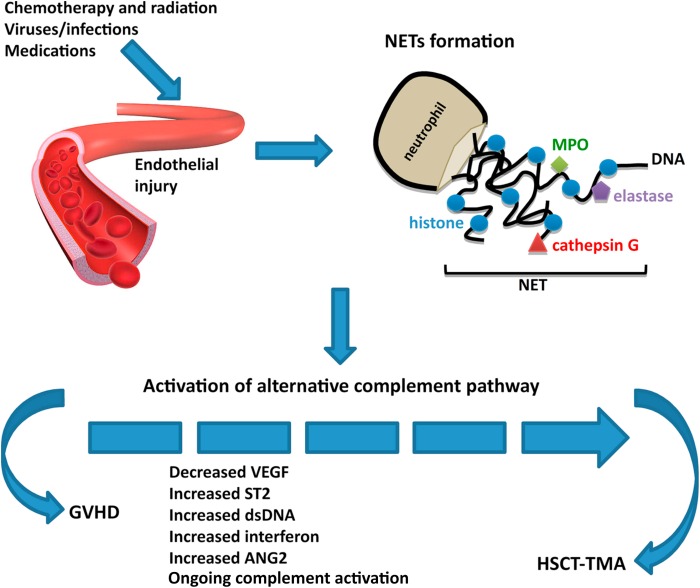
 The complement system can be attacked to help treat kidney disease. Complement activation contributes to the pathogenesis of acute and chronic kidney disease injury. The aHUS and C3GN story has led us to believe that there might be hope for other potential targets in the complement system for patients with kidney disease.
The complement system can be attacked to help treat kidney disease. Complement activation contributes to the pathogenesis of acute and chronic kidney disease injury. The aHUS and C3GN story has led us to believe that there might be hope for other potential targets in the complement system for patients with kidney disease.





 An interesting concept in science is biomimicry. It is the science that takes inspiration from unique designs and processes in nature to help humans.
An interesting concept in science is biomimicry. It is the science that takes inspiration from unique designs and processes in nature to help humans.