This is a schematic of the treatment of resistant HTN in the hemodialysis patient. Based on a review published in JASN
Figure made using biorender.com
This is a schematic of the treatment of resistant HTN in the hemodialysis patient. Based on a review published in JASN
Figure made using biorender.com
As vaccines are arriving at a rapid rate (historic) for SARs-COv2, most of the United States is still dealing with a larger more deadlier wave of infections. Hospitals at most of the US are again at a standstill with what we had seen in March, April in NY.
mRNA vaccine.. we are not used to that technology in the medical world. While reading more on this topic, I found this simplified version by Dr Daniel Goldstein, CT surgeon at Montefiore and a well known voice of COVID care on Linkedin. I have made some changes and additions to his thoughts.
mRNA Vaccines: A primer
The process, simplified:
1. Use DNA, enzymes to create the mRNA sequence that codes for part of SARSCoV2 spike protein
2. Attach 5’cap, poly-A tail and UTRs for stability and better translation
3. Purify and get rid of reagents, enzymes other additives
4. Encapsulate in lipid nanoparticle (phospholipids, PEG, cholesterol) to protect and facilitate delivery into cells.
5. Store in cold (or extremely cold) until use
6. Inject intramuscularly (2 shots, 3-4 wks apart)
7. Encapsulated mRNA taken up by muscles cells.
8. mRNA released into cytoplasm where protein building machinery (ribosomes) will bind to it sequentially and produce many spike proteins. Average 20 sec - couple of mins to make one protein
9. mRNA has half-life about 10 hrs. Sufficient to make lots of protein. Eventually broken down by RNAses.
10. Protein is bound to cell surface where it is recognized as foreign by immune system
11. Ab production, and Ag specific memory B cells and T follicular helper cells are produced
12. More robust response of the above with 2nd injection as body has been “primed”
Advantages of mRNA vaccine:
1. Non-infectious
2. Doesn’t insert into DNA (nucleus).
3. Half life, immunogenicity and delivery can be regulated
4. Quick to make
Disadvantages to me: Seem none, except it's a new technology.
Well we are in a pandemic with a new deadly virus- I would roll those shirts and get the vaccine. What is the data on our renal patients.- Essentially none.
ESKD patients:
To my knowledge, ESKD patients were not in the large vaccine trials but these are vulnerable populations. The UK released a statement of the patients who are most vulnerable in nephrology.
Renal Transplant patients:
Although initial clinical trials of COVID-19 vaccines did not include immunosuppressed patients, we would expect the vaccines to offer protection against COVID-19 infection in these extremely vulnerable patients. An effective COVID-19 vaccine should reduce staff and patient infection resulting in lower rates of serious illness and death. What is interesting as few studies done during the pandemic showed that the renal transplant patients do have a good immune response to the virus( not a lowered one). Studies from Germany and the US showed decent antibody converting. This suggests that vaccines would work in the organ transplant patients and provide amazing protection.
CKD and patients with autoimmune glomerular diseases: No data exists but vaccines would be helpful here as well.
Nephrology community awaits the arrival of the vaccines...
As we enter the end of 2020( finally), we are starting to see some hope for the vaccines as a lifeline as we enter the rising COVID-19 surge. For nephrology, 2020 has been a positive and negative year.
Let's start with the negatives:
1. Covid19 led to development of more AKI than we had imagined and several of those patients dying as a result. Very few survived the RRT-related AKI
2. Our dialysis patients had a tough battle leading to an increased mortality
3. Many transplant centers were on hold and several on the wait list had a high mortality and so did some of our transplant patients.
4. All conferences and meetings were virtual( taking away the networking opportunity for many)
5. All fellowship interviews went virtual( hard to assess candidates candidly)
6. Research ( non covid19) came to a halt and or was interrupted
But there is a silver lining to the COVID19 pandemic for nephrology:
1. Increased data and outcomes research on AKI as a result of the pandemic
2. Rise of HOME dialysis ( which was dormant for years) came more to the forefront( including acute PD)
3. Rise of the Nephrologists as front line COVID19 warriors leading to perhaps more applications this year
4. SGLT2i studies infiltrating NEJM multiple times making a mark on diabetic and non diabetic kidney disease
5. Novel therapeutics in autoimmune renal diseases are on a rise
6. Virtual conferences allowed for more quicker and swifter transfer of knowledge ( and more attendance)
7. Collaboration on research rose super fast with trials such as STOP-COVID
8. Gender and Ethnic diversity was evident in Kidney week this year and kept it's strength in 2020
9. More incentives and compensations increases for nephrologists will reign in 2021
10. Increase interest in subspecialization in Nephrology
While we saw several rising cases of AKI associated with COVID-19, the ESKD population was also vulnerable to this virus. With COVID-19, we didn't know if we would see worsening effects on ESRD or beneficial ( given a not so robust immune system in ESRD). But the proximity and being in a closed dialysis unit did put most of them at risk.
Studies from China and Europe on ESKD patients with COVID-19 were limited to small numbers and single centers. One of the first studies from US from CUMC was limited by less then 100 patients as well. It did show poor outcomes of 59 patients where 31% had died.

A Study from UK did discuss the concerns for an urban dialysis center ( on risk of hospitalizations). Of 1530 patients (median age 66 years; 58.2% men) receiving dialysis, 300 (19.6%) developed COVID-19 infection, creating a large demand for isolated outpatient dialysis and inpatient beds. An analysis that included 1219 patients attending satellite dialysis clinics found that older age was a risk factor for infection. COVID-19 infection was substantially more likely to occur among patients on in-center dialysis compared with those dialyzing at home.
A study from the Bronx in NY also showed poor outcomes for hospitalized ESKD patients. Elevated inflammatory markers were associated with in hospital death.
Another UK study also found a high prevalence of seropositivity in the outpatient dialysis units.

Alberici et al.describe their clinical experience with MHD patients cared for at 4 outpatient dialysis facilities that are part of the Brescia Renal COVID Task Force. In a period of 1 month, viral positivity was detected in 94 of their 643 ESRD HD patients (15%). Important findings in the study were the mild form of symptomatology at presentation, the high rate of overall mortality (29%), and emergence of usual risk factors for mortality and acute respiratory distress syndrome in SARS-CoV-2–positive HD patients. In addition, although certain patients were deemed more stable and were managed in the outpatient facility, 3 of those subsequently died, and a substantial portion had significant worsening of their symptoms.

At our health system of over 23 hospitals in NY, we decided to compare the outcomes of ESKD patients to non ESKD patients. The data was from 13 hospitals and our final cohort had 419 (4%) with ESKD and 10,063 (96%) without ESKD.This is the largest study to date.

What did we find:( similar tweetorial by first author Jia Ng)
I want to share our new paper “Outcomes of patients with ESKD hospitalized with COVID-19”. @kidney_int @hofstrakidney @hofstramed @jam_hirsch @rimdamyeloma @mala_sachdeva @sfishbane @kdjhaveri Susana Hong
— Jia Hwei Ng, MD, MSCE (@jiahweing) August 16, 2020
https://t.co/B2jd3RO4lt
[THREAD] pic.twitter.com/4S03hTRHAo
1. Patients with ESKD were older, had a greater percentage self-identified as Black, and more comorbid conditions.
4. The odds of length of stay of seven or more days was higher in the group with compared to the group without ESKD in both the crude (1.62, 95%CI 1.27 - 2.06) and in the adjusted analysis (1.57, 95% 1.22 - 2.02)
5. We conducted stratified analyses to investigate the risk
factors of death in the subgroups of ESKD and the non-ESKD separately, with the
hypothesis that the risk factors of death and the magnitude of risk factors
would differ between the two groups.
6. For patients without ESKD, the independent risk factors
for in-hospital death increased age, male sex, cardiovascular disease, cancer,
requiring ventilation, requiring vasoactive meds, high blood urea nitrogen, low
albumin, high CRP and high ferritin.
7. The diagnosis of hypertension and use of an ACE inhibitor
or ARB were associated with a lower risk of in-hospital death in the non-ESKD
group.
8. Among patients with ESKD, independent risk factors for
in-hospital death were increased age, requiring ventilation and lymphopenia,
elevated BUN and high serum ferritin. Black race was associated with a
significantly lower risk of death among patients with ESKD.
9. The protective effect of HTN in the non-EKSD group, and
the protective effect of Black race in the ESKD group defy easy explanation. Perhaps APOL1 has some protective cardiac effect?
10. This is a large cohort of hospitalized
patients with #COVID-19 comparing ESKD and non-ESKD in a diverse patient population.
We had prespecified operational definitions for exposures, covariates and
outcomes, as well as rigorous adjudication by two independent reviewers for
ESKD exposure.
11. What limitations do we have?--Despite the larger size of this study compared to other reports, the ESKD sample may still have been relatively underpowered to find other statistically significant risk factors in mortality. Also there was inability to adjust for remdesivir and dexamethasone. As the evidence of these 2 drugs came after the surge of #COVID-19 cases in our health system, only a small proportion of patients received these drugs.
12. We had 11 PD patients in our admitted cohort. This was also published in a special report as well. Of 419 hospitalized patients with ESKD, 11 were on chronic PD therapy (2.6%). Among those 11, 3 patients required mechanical ventilation, 2 of whom died. Of the entire cohort, 9 of the 11 patients (82%) were discharged alive. While fever was a common presentation, more than half of our patients also presented with diarrhea. Interestingly, 3 patients were diagnosed with culture-negative peritonitis during their hospitalization. Seven patients reported positive SARS-CoV-2 exposure from a member of their household.
In conclusion, among patients hospitalized with COVID-19, those with ESKD had a higher rate of in-hospital death compared to those without ESKD.
Two recent studies also show the outpatient HD infection and admission rates. A study published in AJKD from Canada showed from universal screening, 4.6% were infected.
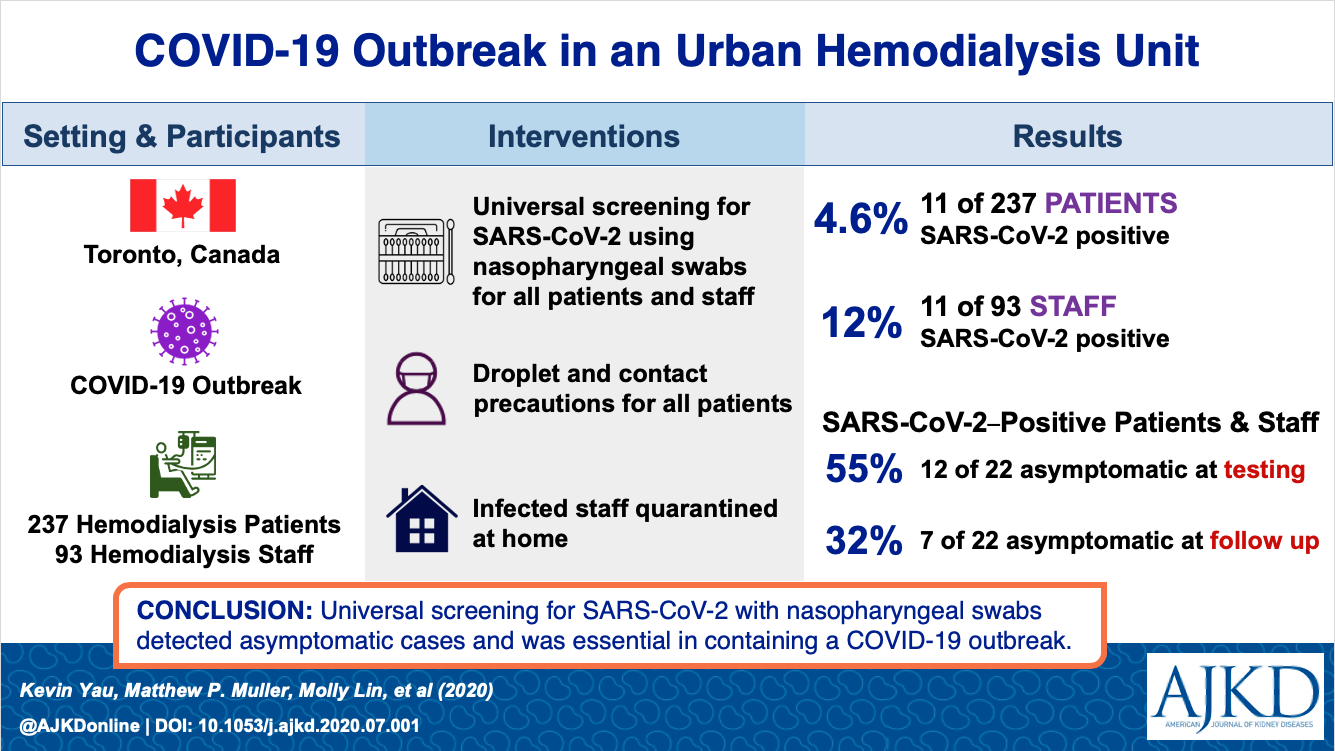
Another French study in KI showed a low incidence of infection of 3.3% in a large >40,000 dialysis patients. Older age, low albumin, and cardiac disease were risk factors for mortality.

Taken together, the results suggest both a need for further research and the continued need for careful infection control procedures in the ESKD population at risk for #COVID-19.
Class
|
%Removal with
hemodialysis |
||
Angiotensin converting enzyme inhibitors
|
|||
Captopril
|
Yes
|
||
Benazepril
|
20–50%
|
||
Enalapril
|
35%
|
||
Fosinopril
|
<10%
|
||
Lisinopril
|
50%
|
||
Quinapril
Ramipril |
<10%( limited data)
<30% |
||
Angiotensin receptor blockers
|
|||
Losartan
|
None
|
||
Candesartan
|
None
|
||
Eprosartan
|
None
|
||
Telmisartan
|
None
|
||
Valsartan
|
None
|
||
Irbesartan
|
None
|
||
Aldosterone antagonists
|
|||
Spironolactonea
|
None
|
||
Eplerenoneb
|
None
|
||
Renin inhibitor
|
|||
Aliskiren
|
?
|
||
β-Blockers and combined α- and β-blockers
|
|||
Atenolol
|
75%
|
||
Metoprolol
|
High
|
||
Metoprolol XL
|
High
|
||
Propranolol
|
<5%
|
||
Carvedilol
|
None
|
||
Carvedilol CR
|
None
|
||
Labetalol
|
<1%
|
||
Calcium channel blockers
|
|||
Amlodipine
|
None
|
||
Diltiazem
|
<30%
|
||
Nifedipine
|
Low
|
||
Nicardipine
|
?
|
||
Felodipine
|
No
|
||
Verapamil
|
Low
|
||
Alpha-adrenergic blockers
|
|||
Doxazosinc
|
None
|
||
Terazosin
|
None
|
||
Prazosin
|
?
|
||
Other
|
|||
Clonidine
|
<5%
|
||
Hydralazine
|
None
|
||
Isosorbide dinitrate
|
Yes
|
||
Minoxidil
|
Partially
|
